Question 1
Complete the following table
| Subatomic partical | Symbol | Relatitive mass | Relative charge |
| Electron | |||
| Neutron | |||
| Proton |
Easy
Mark as Complete
Mark Scheme
Question 2
Define the term relative atomic mass of an element.
Medium
Mark as Complete
Mark Scheme
Question 3
What is the main difference between atomic number and nucleon number?
Easy
Mark as Complete
Mark Scheme
Question 4
Complete the table
| Neutral atom | Atomic number | Nucleon number | Numbers of each subatomic particle present |
| Mg | 12 | 24 | |
| U | 92 | 235 |
Easy
Mark as Complete
Mark Scheme
Question 5
When calculating the relative mass of an atom, are the electrons used in the calculation or not? Why?
Easy
Mark as Complete
Mark Scheme
Question 6
The subatomic particles present in zirconium and hafnium are electrons, neutrons and protons. A beam of protons is fired into an electric field produced by two charged plates, as shown in the diagram.
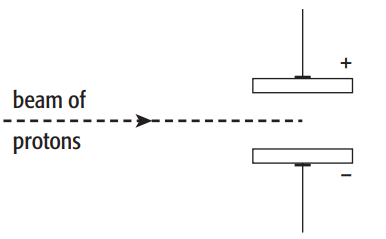
a. Describe how the beam of protons behaves when it passes through the gap between the charged plates.
b. Explain your answer.
Hard
Mark as Complete
Mark Scheme
Question 7
Magnesium chloride contains magnesium ions, `Mg^(2+)`, and chloride ions, `Cl^-`.
Easy
Mark as Complete
Mark Scheme
Question 8
An aluminum atom has 13 protons in its nucleus. Explain why it cannot have 14 protons
Medium
Mark as Complete
Mark Scheme
Question 9
In which species are the numbers of protons, neutrons and electrons all different?
A. `""_13^27Al`
B. `""_17^37Cl^-`
C. `""_16^32S^(2-)`
D. `""_19^39K^+`
Medium
Mark as Complete
Mark Scheme
Question 10
Using the periodic table to answer this question
Which statement about the phosphide ion, `"^31P^(3-)`, and the chloride ion, `"^37Cl^-`, are correct?
A. They have the same number of electrons.
B. They have the same number of neutrons.
C. They have the same number of protons.
D. They have the same number of nucleons.
Medium
Mark as Complete
Mark Scheme
Question 1
Complete the following table
| Subatomic partical | Symbol | Relatitive mass | Relative charge |
| Electron | |||
| Neutron | |||
| Proton |
| Subatomic partical | Symbol | Relatitive mass | Relative charge |
| Electron | e | `frac{1}{1836}` | -1 |
| Neutron | n | 1 | 0 |
| Proton | p | 1 | +1 |
Question 2
Define the term relative atomic mass of an element.
That is how heavy one atom is compared with another. More specifically, it refers to the weighted average mass of an atom of the element, relative to 1/12th the mass of a carbon-12 atom.
Question 3
What is the main difference between atomic number and nucleon number?
The atomic number is the number of the protons in the nucleus whereas the nucleon number comprise the total number of protons and neutrons in an atom
Question 4
Complete the table
| Neutral atom | Atomic number | Nucleon number | Numbers of each subatomic particle present |
| Mg | 12 | 24 | |
| U | 92 | 235 |
Mg = 12 protons, 12 electrons, 12 neutrons
U = 92 protons, 92 electrons, 143 neutrons
Question 5
When calculating the relative mass of an atom, are the electrons used in the calculation or not? Why?
The electrons are not used in the calculation because the mass of an electron is almost nothing in comparison to protons. It’s just only `frac{1}{1836}`the mass of a proton. Therefore, the mass of an electron is negligible
Question 6
The subatomic particles present in zirconium and hafnium are electrons, neutrons and protons. A beam of protons is fired into an electric field produced by two charged plates, as shown in the diagram.

a. Describe how the beam of protons behaves when it passes through the gap between the charged plates.
b. Explain your answer.
a. The protons would be attracted to the negative plate and repelled from the positive plate, the beam would bend in the direction of the negative plate as protons are positively charged.
b. Like charges repels and unlike charges attracts each other.
Question 7
Magnesium chloride contains magnesium ions, `Mg^(2+)`, and chloride ions, `Cl^-`.
The magnesium ion has a charge of 2+ because it has 12 protons (+) but only 10 electrons (–).
The chloride ion has a single negative charge because there are 17 protons (+) and 18 electrons (–).
Question 8
An aluminum atom has 13 protons in its nucleus. Explain why it cannot have 14 protons
An aluminum atom is described as an atom with 13 protons found in the nucleus (atom weight of 27). If hypothetically, an aluminum atom contained 14 protons, it would no longer be considered an "aluminum" atom. Once an aluminum atom has 14 protons it is considered a "silic" atom.
Question 9
In which species are the numbers of protons, neutrons and electrons all different?
A. `""_13^27Al`
B. `""_17^37Cl^-`
C. `""_16^32S^(2-)`
D. `""_19^39K^+`
The answer is D.
`""_13^27Al` = 13 protons, 13 electrons, 14 neutrons.
`""_17^37Cl^-`= 17 protons, 18 electrons, 18 neutrons
`""_16^32S^(2-)`= 16 protons, 18 electrons, 16 neutrons
`""_19^39K^+`= 19 protons, 18 electrons, 20 neutrons
Question 10
Using the periodic table to answer this question
Which statement about the phosphide ion, `"^31P^(3-)`, and the chloride ion, `"^37Cl^-`, are correct?
A. They have the same number of electrons.
B. They have the same number of neutrons.
C. They have the same number of protons.
D. They have the same number of nucleons.
The answer is A.
`"^31P^(3-)`= 15 protons, 18 electrons, 16 neutrons
`"^35Cl^-` = 17 protons, 18 electrons, 18 neutrons
Question 1
Complete the following table
| Subatomic partical | Symbol | Relatitive mass | Relative charge |
| Electron | |||
| Neutron | |||
| Proton |
Question 2
Define the term relative atomic mass of an element.
Question 3
What is the main difference between atomic number and nucleon number?
Question 4
Complete the table
| Neutral atom | Atomic number | Nucleon number | Numbers of each subatomic particle present |
| Mg | 12 | 24 | |
| U | 92 | 235 |
Question 5
When calculating the relative mass of an atom, are the electrons used in the calculation or not? Why?
Question 6
The subatomic particles present in zirconium and hafnium are electrons, neutrons and protons. A beam of protons is fired into an electric field produced by two charged plates, as shown in the diagram.

a. Describe how the beam of protons behaves when it passes through the gap between the charged plates.
b. Explain your answer.
Question 7
Magnesium chloride contains magnesium ions, `Mg^(2+)`, and chloride ions, `Cl^-`.
Question 8
An aluminum atom has 13 protons in its nucleus. Explain why it cannot have 14 protons
Question 9
In which species are the numbers of protons, neutrons and electrons all different?
A. `""_13^27Al`
B. `""_17^37Cl^-`
C. `""_16^32S^(2-)`
D. `""_19^39K^+`
Question 10
Using the periodic table to answer this question
Which statement about the phosphide ion, `"^31P^(3-)`, and the chloride ion, `"^37Cl^-`, are correct?
A. They have the same number of electrons.
B. They have the same number of neutrons.
C. They have the same number of protons.
D. They have the same number of nucleons.