Question 1
Animal cells often secrete glycoproteins as extracellular components. What is a role of these glycoproteins?
A. Adhesion
B. Additional energy reserve
C. Membrane fluidity
D. Water uptake
Medium
Mark as Complete
Mark Scheme
Question 2
Which are functions of lipids?
A. Hydrophilic solvent and energy storage.
B. Hydrophobic solvent and membrane potential.
C. Thermal insulation and energy storage.
D. Thermal insulation and hydrophilic solvent
Easy
Mark as Complete
Mark Scheme
Question 3
Which types of molecule are shown in the diagrams?
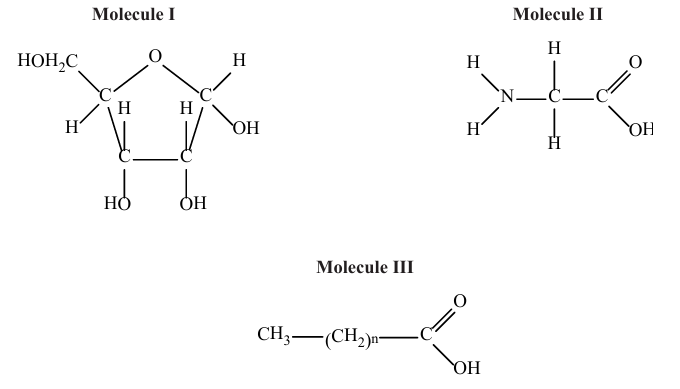
| Molecule I | Molecule II | Molecule III | |
| A. | amino acid | fatty acid | ribose |
| B. | glucose | amino acid | fatty acid |
| C. | ribose | amino acid | fatty acid |
| D. | fatty acid | glucose | amino acid |
Easy
Mark as Complete
Mark Scheme
Question 4
Which type (s) of fatty acid in the diet is/are positively correlated with an increased risk of coronary heart disease?
I. Saturated
II. Trans unsaturated
III. Cis unsaturated
A. I only
B. I and II only
C. II only
D. II and III only
Easy
Mark as Complete
Mark Scheme
Question 5
Which statement applies to cholesterol?
A. It is hydrophobic and found on the outside of the phospholipid bilayer.
B. It is hydrophilic and found inside the phospholipid bilayer.
C. It impacts membrane fluidity.
D. It is transported in association with glucose in the blood.
Medium
Mark as Complete
Mark Scheme
Question 6
Which part of the membrane allows cell recognition?
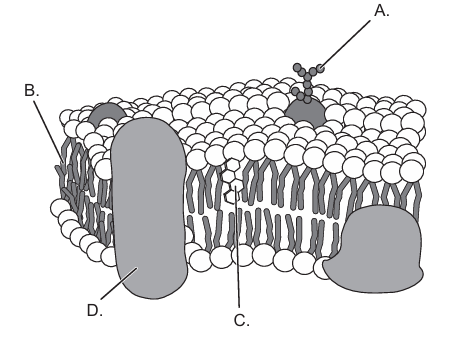
Medium
Mark as Complete
Mark Scheme
Question 7
Testosterone is a hormone that is important for male reproductive development.
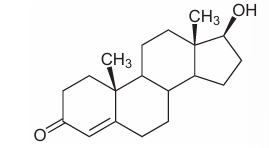
To which group of compounds does testosterone belong?
A. Nucleotides
B. Carbohydrates
C. Lipids
D. Amino acid
Easy
Mark as Complete
Mark Scheme
Question 8
Which features of phospholipids give them their amphipathic properties?
A. Basic phosphate groups and acidic lipids
B. Acidic phosphate groups and basic lipids
C. Hydrophobic phosphate groups and hydrophilic fatty acids
D. Hydrophilic phosphate groups and hydrophobic fatty acids
Easy
Mark as Complete
Mark Scheme
Question 9
Outline how the amphipathic properties of phospholipids help determine membrane structure. [4]
Medium
Mark as Complete
Mark Scheme
Question 10
Outline the role of condensation and hydrolysis in metabolic reactions involving carbohydrates. [4]
Medium
Mark as Complete
Mark Scheme
Question 1
Animal cells often secrete glycoproteins as extracellular components. What is a role of these glycoproteins?
A. Adhesion
B. Additional energy reserve
C. Membrane fluidity
D. Water uptake
Answer: A
A. Correct. Many animal cells are able to adhere to one another, and this adhesion is mediated by glycoproteins that the cells secrete.
B. Incorrect. In living organisms, polysaccharides such as glycogen are used as energy storage molecules in animals. Lipids (fats) are also used for long-term energy storage.
C. Incorrect. Membrane fluidity is mainly regulated by other factors such as the fatty acid composition of phospholipids and the presence of cholesterol. Cholesterol acts as a modulator of membrane fluidity, stabilizing the membrane at higher temperatures and preventing it from becoming too rigid at lower temperatures.
D. Incorrect. Water uptake occurs through osmosis. In cells, water moves through the phospholipid bilayer and through special channel proteins called aquaporins. Although the glycocalyx (which contains glycoproteins) is highly hydrophilic and attracts a large amount of water to the cell surface, its most clearly defined roles are recognition and adhesion.
Question 2
Which are functions of lipids?
A. Hydrophilic solvent and energy storage.
B. Hydrophobic solvent and membrane potential.
C. Thermal insulation and energy storage.
D. Thermal insulation and hydrophilic solvent
Answer: C
A. Incorrect. Lipids are described as hydrophobic. Water is the main hydrophilic solvent essential for life.
B. Incorrect. Lipids are hydrophobic. However, in a biological context, fats (triglycerides) do not function as solvents. The membrane potential is related to ion concentration gradients maintained by protein pumps, such as the sodium–potassium pump. Although phospholipids are fundamental components of membranes and cholesterol regulates membrane fluidity, lipids (triglycerides/fats) are not defined as having the function of maintaining membrane potential.
Membrane potential comes from ion gradients maintained by protein pumps and ion channels, not by lipids.
C. Correct. Triglycerides (a type of lipid) are used for energy storage in plants and animals, and they release more than twice as much energy per mass as carbohydrates. Lipids are poor conductors of heat, so they can act as thermal insulators. For example, large fat deposits in marine mammals serve as insulation against heat loss
D. Incorrect. Thermal insulation is a correct function. However, hydrophilic solvent is incorrect because lipids are hydrophobic.
Question 3
Which types of molecule are shown in the diagrams?

| Molecule I | Molecule II | Molecule III | |
| A. | amino acid | fatty acid | ribose |
| B. | glucose | amino acid | fatty acid |
| C. | ribose | amino acid | fatty acid |
| D. | fatty acid | glucose | amino acid |
Answer: C
I. The structure of Molecule I shows Ribose, a pentose sugar (a 5-carbon sugar) in ring form.
II. The structure of Molecule II shows general structure of an Amino acid. It has central carbon atom (the α-carbon) bonded to an amino group (N and H), a carboxyl group (C, O, OH), a hydrogen atom, and a side chain (R-group, shown here as H, which may represent a simple R group).
III.The structure of Molecular III is represented as `CH_3(CH_2)_nCOOH`. This structure describes a long hydrocarbon chain (`CH_3(CH_2)_nCOOH`) ending with a carboxyl group (COOH), which is a definition of a Fatty acid.
Question 4
Which type (s) of fatty acid in the diet is/are positively correlated with an increased risk of coronary heart disease?
I. Saturated
II. Trans unsaturated
III. Cis unsaturated
A. I only
B. I and II only
C. II only
D. II and III only
Answer: B
I. Diets high in saturated fatty acids (e.g., animal fats) often lead to abnormally high blood cholesterol levels. Excess cholesterol, present mainly as LDL (low-density lipoproteins, also known as “bad cholesterol”), can cause atherosclerosis. Atherosclerosis is the progressive degeneration of arterial walls. A direct consequence of this is an increased likelihood of forming and circulating blood clots, which can lead to strokes and heart attacks.
II. Trans unsaturated fatty acids have straight chains and are produced artificially through partial hydrogenation. Trans fats pose serious health risks and that an oil containing 45% trans fat is considered least recommended implies that they increase the risk of coronary heart disease (CHD).
III.Polyunsaturated fats, typically in the cis form, are considered important for arterial health. One study estimates that replacing saturated fats with polyunsaturated fats in the diet may reduce the risk of cardiovascular disease by about 30%.
Therefore, only I (Saturated) and II (Trans unsaturated) are positively correlated with an increased risk of CHD.
Question 5
Which statement applies to cholesterol?
A. It is hydrophobic and found on the outside of the phospholipid bilayer.
B. It is hydrophilic and found inside the phospholipid bilayer.
C. It impacts membrane fluidity.
D. It is transported in association with glucose in the blood.
Answer: C
A. Incorrect. Cholesterol is a steroid. It is an amphipathic molecule because it has a hydrophilic hydroxyl group (-OH) and a largely hydrophobic structure. Its position is embedded within the phospholipid bilayer (the hydrophobic core), not simply outside it
B. Incorrect. Cholesterol is not completely hydrophilic. Its hydrophilic part (the -OH group) faces outward, interacting with the phosphate heads of phospholipids, while the hydrophobic part is oriented toward the interior of the lipid bilayer.
C. Correct. Cholesterol is a component of the cell membrane and regulates membrane fluidity. It stabilizes the membrane at higher temperatures and prevents it from becoming too rigid at lower temperatures.
D. Incorrect. Glucose is a polar, water-soluble molecule that is transported in blood plasma. Cholesterol is transported in the blood as part of lipoprotein complexes (low-density lipoproteins—LDL and high-density lipoproteins—HDL). It is not transported together with glucose.
Question 6
Which part of the membrane allows cell recognition?

Answer: A
A. Correct. This is the carbohydrate chain on a glycoprotein. Carbohydrate chains on the outer surface of the membrane allow cell recognition
B. Incorrect. This is a phospholipid and it doees not function in a cell recognition. While membrane proteins have many roles, cell recognition requires carbohydrates
C. Incorrect. This is a transmembrane protein without a carbohydrate chain..
D. Incorrect. Transport proteins move molecules but do not provide recognition.
Question 7
Testosterone is a hormone that is important for male reproductive development.

To which group of compounds does testosterone belong?
A. Nucleotides
B. Carbohydrates
C. Lipids
D. Amino acid
Answer: C
A. Incorrect. Nucleotides include ATP, DNA/RNA monomers; their structures include a sugar, phosphate, and nitrogenous base — nothing like testosterone.
B. Incorrect.. Carbohydrates are ring-shaped sugars (mono-, di-, polysaccharides). Testosterone is not a sugar
C. Correct. Testosterone is a steroid, and steroids are classified as lipids. They are hydrophobic and derived from cholesterol.
D. Incorrect. Amino acids contain an amino group, carboxyl group, and an R-group. Testosterone has none of these features.
Question 8
Which features of phospholipids give them their amphipathic properties?
A. Basic phosphate groups and acidic lipids
B. Acidic phosphate groups and basic lipids
C. Hydrophobic phosphate groups and hydrophilic fatty acids
D. Hydrophilic phosphate groups and hydrophobic fatty acids
Answer: D
A. Incorrect. Although fatty acids are weakly acidic (due to their ionizable carboxyl group), classifying the head as “basic” and the tail as “acidic” is not an accurate description of the hydrophilic/hydrophobic properties that give rise to amphipathicity.
B. Incorrect. Lipids (fatty acids) are hydrophobic.
C. Incorrect. This reverses the actual properties: the phosphate head is hydrophilic, and the fatty acid tails are hydrophobic.
D. Incorrect. This option accurately describes the combination of a hydrophilic (phosphate) head and hydrophobic (fatty acid) tails, which gives phospholipids their amphipathic nature.
Question 9
Outline how the amphipathic properties of phospholipids help determine membrane structure. [4]
Any four of the following:
a. membrane structure is double layer of phospholipids / bilayer;
b. phospholipids consist of a phosphate head and 2 fatty acid tails;
c. the (phosphate) head is polar / hydrophilic;
d. the (fatty acid) tails are non-polar / hydrophobic;
e. tails repel water/ are attracted to each other so found towards the inside (of the bilayer);
f. head forms H bonds/ interacts with water so face outwards (of the bilayer);
Sample answer:
The amphipathic properties of phospholipids cause them to arrange themselves into a stable bilayer that forms the basic structure of cell membranes. Each phospholipid has a hydrophilic phosphate head [1] and two hydrophobic fatty acid tails [1]. Because the heads are attracted to water while the tails avoid it, the molecules align so that the hydrophilic heads face outward [1] toward the aqueous internal and external environments. At the same time, the hydrophobic tails cluster together [1], away from water, forming the interior of the bilayer. This natural self-organization creates the double-layered membrane [1] that is both stable in water and flexible enough to support membrane proteins and dynamic cell functions.
Question 10
Outline the role of condensation and hydrolysis in metabolic reactions involving carbohydrates. [4]
Any four of the following:
a. condensation is joining together molecules with the release of water;
b. (in general) two monosaccharides join to form a disaccharide / many mono-saccharides/disaccharides form polysaccharides;
c. example; (eg two glucose from maltose)
d. hydrolysis is the breaking down of molecules with the addition of water;
e. (in general) disaccharides break into monosaccharides / polysaccharides break into disaccharides/monosaccharides;
f. example; (eg maltose forms two glucose)
Sample answer:
Condensation and hydrolysis play opposite but complementary roles in carbohydrate metabolism. Condensation reactions join molecules together while releasing water [1], allowing two monosaccharides to form a disaccharide or many monosaccharides to build polysaccharides [1]. For example, two glucose molecules can join by condensation to form maltose. In contrast, hydrolysis breaks larger carbohydrate molecules apart by adding water [1], such as when a disaccharide splits into its monosaccharide components [1]. An example is the hydrolysis of maltose to produce two glucose molecules. These paired processes allow organisms to build energy-storage molecules and later break them down when energy is needed.
Question 1
Animal cells often secrete glycoproteins as extracellular components. What is a role of these glycoproteins?
A. Adhesion
B. Additional energy reserve
C. Membrane fluidity
D. Water uptake
Question 2
Which are functions of lipids?
A. Hydrophilic solvent and energy storage.
B. Hydrophobic solvent and membrane potential.
C. Thermal insulation and energy storage.
D. Thermal insulation and hydrophilic solvent
Question 3
Which types of molecule are shown in the diagrams?

| Molecule I | Molecule II | Molecule III | |
| A. | amino acid | fatty acid | ribose |
| B. | glucose | amino acid | fatty acid |
| C. | ribose | amino acid | fatty acid |
| D. | fatty acid | glucose | amino acid |
Question 4
Which type (s) of fatty acid in the diet is/are positively correlated with an increased risk of coronary heart disease?
I. Saturated
II. Trans unsaturated
III. Cis unsaturated
A. I only
B. I and II only
C. II only
D. II and III only
Question 5
Which statement applies to cholesterol?
A. It is hydrophobic and found on the outside of the phospholipid bilayer.
B. It is hydrophilic and found inside the phospholipid bilayer.
C. It impacts membrane fluidity.
D. It is transported in association with glucose in the blood.
Question 6
Which part of the membrane allows cell recognition?

Question 7
Testosterone is a hormone that is important for male reproductive development.

To which group of compounds does testosterone belong?
A. Nucleotides
B. Carbohydrates
C. Lipids
D. Amino acid
Question 8
Which features of phospholipids give them their amphipathic properties?
A. Basic phosphate groups and acidic lipids
B. Acidic phosphate groups and basic lipids
C. Hydrophobic phosphate groups and hydrophilic fatty acids
D. Hydrophilic phosphate groups and hydrophobic fatty acids
Question 9
Outline how the amphipathic properties of phospholipids help determine membrane structure. [4]
Question 10
Outline the role of condensation and hydrolysis in metabolic reactions involving carbohydrates. [4]