Question 1
Cell metabolism involves anabolic and catabolic reactions. Which process directly involves anabolism?
A. Active transport of ions.
B. Release of energy from glucose.
C. Production of intracellular enzymes.
D. Breakdown of worn-out cell organelles by lysosomes.
Easy
Mark as Complete
Mark Scheme
Question 2
The graph shows the effect of temperature on the rate of a chemical reaction catalysed by enzymes.
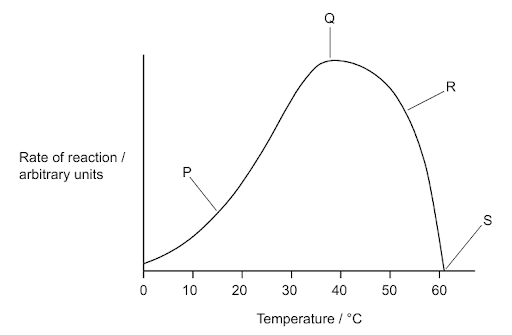
What is a valid statement about a labelled point in the graph?
A. At P, substrate concentration is limiting the rate of reaction.
B. At Q, substrate and enzyme molecules achieve their highest kinetic energy.
C. At R, some active sites have changed shape.
D. At S, all substrate molecules have formed product.
Easy
Mark as Complete
Mark Scheme
Question 3
The diagram shows the formation of an enzyme–substrate complex.
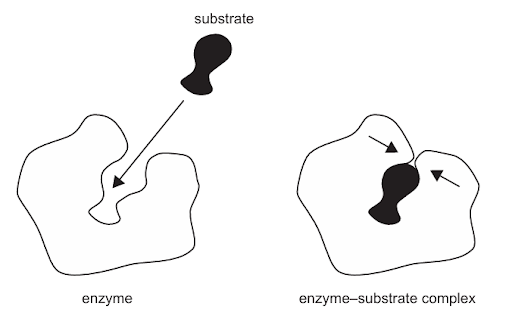
What describes the process shown in the diagram?
A. The lock and key hypothesis, as the substrate is complementary to the enzyme.
B. The substrate permanently alters the shape of the enzyme’s active site.
C. The substrate and the active site have the same shape.
D. The active site changes shape to accommodate the substrate in the induced-fit model.
Easy
Mark as Complete
Mark Scheme
Question 4
Which statement applies to enzymes?
A. Enzyme function depends on collisions between substrate and active sites.
B. One active site typically binds to a broad range of substrates.
C. The active site on the substrate is specific to one enzyme.
D. When enzymes are immobilized they stop working.
Easy
Mark as Complete
Mark Scheme
Question 5
What effect do changes in pH have on enzymes?
A. All enzymes increase in activity as pH increases.
B. The activity of all enzymes is reduced by a pH below or above 7.
C. Low pH causes reversible denaturation in all enzymes.
D. Extreme pH can alter the active site of all enzymes.
Easy
Mark as Complete
Mark Scheme
Question 6
Which products are formed by the action of the enzymes protease and amylase?
| Protease | Amylase | |
| A. | fatty acids | glucose |
| B. | glycerol | fatty acids |
| C. | proteins | starch |
| D. | amino acids | maltose |
Easy
Mark as Complete
Mark Scheme
Question 7
A fever in a normally healthy adult during an illness is not usually a problem and can be regarded as a defence mechanism. However, a fever higher than 41°C might be dangerous. What is the cause of the possible damage due to a high fever?
A. Loss of body mass
B. Muscle damage due to shivering
C. Overactive metabolic enzymes
D. Spread of infection
Easy
Mark as Complete
Mark Scheme
Question 8
Some bacteria can synthesize the amino acid isoleucine from threonine, a process involving five enzymes (E1 to E5) and four intermediary products (P, Q, R and S). The production of isoleucine is controlled by end-product inhibition.
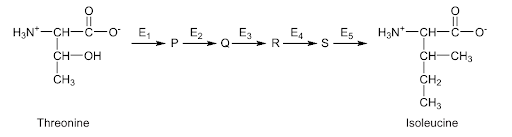
Which statement describes this end-product inhibition?
A. If isoleucine accumulates, it inhibits the production of P.
B. End-product inhibition causes a build-up of intermediary products.
C. Isoleucine inhibits E5, so no more isoleucine is produced.
D. Isoleucine affects the structure of threonine.
Easy
Mark as Complete
Mark Scheme
Question 9
Explain how temperature affects enzymes. [4]
Hard
Mark as Complete
Mark Scheme
Question 10
Outline the action of enzymes. [4]
Hard
Mark as Complete
Mark Scheme
Question 11
Explain the regulation of metabolic pathways by end-product inhibition. [4]
Hard
Mark as Complete
Mark Scheme
Question 1
Cell metabolism involves anabolic and catabolic reactions. Which process directly involves anabolism?
A. Active transport of ions.
B. Release of energy from glucose.
C. Production of intracellular enzymes.
D. Breakdown of worn-out cell organelles by lysosomes.
Answer: C
A. Incorrect: Active transport moves ions across membranes using ATP, but it does not build or synthesize molecules. This process is a cellular activity powered by energy, not an anabolic or catabolic reaction itself. It’s part of cell physiology, not metabolism’s building or breaking reactions.
B. Incorrect: Releasing energy from glucose is a catabolic process (cellular respiration). Catabolism breaks down complex molecules (like glucose) into simpler ones (like CO₂ and H₂O), releasing energy in the process. This is the opposite of anabolism.
C. Correct: Anabolism refers to the synthesis (building up) of complex molecules from simpler ones. The production of intracellular enzymes means forming large protein molecules from amino acids, which is a biosynthetic (anabolic) process. This process requires energy (ATP) to form peptide bonds and build the enzyme structures inside the cell. Therefore, this option correctly describes anabolism, since it involves building complex macromolecules that the cell uses for structure or function.
D. Incorrect: This is a degradative (catabolic) process. Lysosomes contain hydrolytic enzymes that break down macromolecules and organelles into smaller components for recycling. It involves digestion, not synthesis.
Question 2
The graph shows the effect of temperature on the rate of a chemical reaction catalysed by enzymes.

What is a valid statement about a labelled point in the graph?
A. At P, substrate concentration is limiting the rate of reaction.
B. At Q, substrate and enzyme molecules achieve their highest kinetic energy.
C. At R, some active sites have changed shape.
D. At S, all substrate molecules have formed product.
Answer: C
A. Incorrect: The graph shows temperature as the only changing factor, not substrate concentration. At P (low temperature), the rate is low because enzyme and substrate molecules have low kinetic energy, not because substrate is limited.
B. Incorrect: The kinetic energy continues to increase as temperature rises beyond Q. Q represents the optimum temperature, where enzyme activity is highest, but not necessarily the maximum kinetic energy. Beyond Q, even though kinetic energy is higher, the enzyme denatures, causing the reaction rate to fall.
C. Correct: The graph shows the effect of temperature on enzyme activity. As temperature increases, the rate of reaction rises (from P to Q) because molecules gain kinetic energy, leading to more frequent collisions between enzyme and substrate. The rate reaches a maximum at Q, which is the optimum temperature for enzyme activity. Beyond this point (R), the enzyme starts to denature - its active site changes shape, so substrates can no longer bind effectively. Therefore, at R, some of the enzyme’s active sites have been altered by heat, decreasing the reaction rate.
D. Incorrect: The reaction rate drops at S due to enzyme denaturation, not because all substrate has been used. The enzyme’s structure is mostly destroyed at this point, so few or no reactions occur, even if substrate is still present.
Question 3
The diagram shows the formation of an enzyme–substrate complex.

What describes the process shown in the diagram?
A. The lock and key hypothesis, as the substrate is complementary to the enzyme.
B. The substrate permanently alters the shape of the enzyme’s active site.
C. The substrate and the active site have the same shape.
D. The active site changes shape to accommodate the substrate in the induced-fit model.
Answer: D
A. Incorrect: In the lock-and-key model, the active site of the enzyme is the complementary shape of the substrate and so fits to the substrate precisely. In the diagram, the enzyme’s active site changes shape upon binding, which matches the induced-fit model, not lock-and-key.
B. Incorrect: The shape change is temporary and reversible. After the reaction, the enzyme returns to its original shape and can be reused. A permanent change would mean the enzyme is denatured, which doesn’t happen here.
C. Incorrect: The substrate and active site are complementary, not identical. The enzyme’s active site adjusts its shape to fit the substrate; they are not the same shape from the beginning.
D. Correct: The diagram shows an enzyme and substrate before and after forming an enzyme-substrate complex. In the induced-fit model, the enzyme’s active site is flexible, not rigid. When the substrate binds, the active site changes shape slightly to fit the substrate more closely. This ensures a tighter binding, better orientation of reactive groups, and optimal catalysis. The shape change is temporary after the reaction, the enzyme returns to its original form. Therefore, the process in the diagram illustrates the induced-fit model.
Question 4
Which statement applies to enzymes?
A. Enzyme function depends on collisions between substrate and active sites.
B. One active site typically binds to a broad range of substrates.
C. The active site on the substrate is specific to one enzyme.
D. When enzymes are immobilized they stop working.
Answer: A
A. Correct: Enzymes speed up reactions by binding to substrates at their active sites. For this to happen, the substrate molecules must collide with the enzyme’s active site with sufficient energy and in the correct orientation. The rate of enzyme activity depends on how often and how effectively these enzyme–substrate collisions occur. Factors like temperature, substrate concentration, and enzyme concentration affect these collision rates. Therefore, enzyme function indeed depends on collisions between substrate and active sites.
B. Incorrect: Active sites are highly specific. The shape and chemical properties of an active site fit only one (or very few) substrates, like a lock and key. Only in rare cases do enzymes act on similar substrates, but generally specificity is a key feature of enzyme action.
C. Incorrect: Substrates do not have active sites, but enzymes do. The substrate has a binding region or a shape complementary to the enzyme’s active site, but it’s incorrect to say the substrate itself has an active site. This statement confuses the roles of enzyme and substrate.
D. Incorrect: The immobilized enzymes remain active. Immobilization means enzymes are attached to a solid surface (like beads or membranes) to make them easier to separate and reuse. Their catalytic function continues, often with increased stability.
Question 5
What effect do changes in pH have on enzymes?
A. All enzymes increase in activity as pH increases.
B. The activity of all enzymes is reduced by a pH below or above 7.
C. Low pH causes reversible denaturation in all enzymes.
D. Extreme pH can alter the active site of all enzymes.
Answer: D
A. Incorrect: Each enzyme has an optimum pH at which it works best. For example: pepsin (in the stomach) works best at pH 2 (acidic), amylase (in the mouth) works best at pH 7 (neutral). Increasing pH beyond the optimum reduces activity - not increases it.
B. Incorrect: Enzymes adapted to different environments (e.g., stomach vs. intestine) have different pH optima. So while extreme pH affects enzymes, it doesn’t mean pH 7 is ideal for all of them.
C. Incorrect: Denaturation is usually irreversible when the structure is significantly altered. Some minor conformational changes can be reversed if pH returns to normal, but true denaturation (loss of shape of the active site) is irreversible. Also, not all enzymes denature at low pH - only those not adapted to acidic conditions do.
D. Correct: Enzymes are proteins, and their shape (especially the active site) depends on the ionic and hydrogen bonds that maintain their 3D structure. When the pH becomes too high or too low (extreme pH), the H⁺ or OH⁻ ions interfere with these bonds. This can change the shape of the active site, preventing the substrate from binding - a process known as denaturation. Once denatured, the enzyme can no longer catalyze the reaction, because the substrate no longer fits. Therefore, extreme pH alters the active site of enzymes, affecting their function.
Question 6
Which products are formed by the action of the enzymes protease and amylase?
| Protease | Amylase | |
| A. | fatty acids | glucose |
| B. | glycerol | fatty acids |
| C. | proteins | starch |
| D. | amino acids | maltose |
Answer: D
A. Incorrect: Fatty acids are produced by lipase, not protease. Amylase produces maltose, not glucose (glucose comes later when maltose is broken down by maltase).
B. Incorrect: Both glycerol and fatty acids are products of lipid (fat) digestion by lipase, not protease or amylase. Protease acts on proteins, and amylase acts on starch, not fats.
C. Incorrect: These are substrates, not products. Protease acts on proteins to make amino acids. Amylase acts on starch to make maltose.
D. Correct: Protease is an enzyme that breaks down proteins into amino acids. Proteins are long chains of amino acids linked by peptide bonds. Protease hydrolyzes these peptide bonds - producing individual amino acids. Amylase is an enzyme that breaks down starch (a polysaccharide) into maltose (a disaccharide). Starch is composed of many glucose molecules linked together. Amylase acts on starch - producing maltose (two glucose units joined together). Therefore, this option is correct.
Question 7
A fever in a normally healthy adult during an illness is not usually a problem and can be regarded as a defence mechanism. However, a fever higher than 41°C might be dangerous. What is the cause of the possible damage due to a high fever?
A. Loss of body mass
B. Muscle damage due to shivering
C. Overactive metabolic enzymes
D. Spread of infection
Answer: C
A. Incorrect: Although fever can cause mild dehydration and energy use, loss of body mass is not the direct cause of damage. The danger of high fever comes from enzyme denaturation, not weight loss.
B. Incorrect: Shivering occurs when body temperature is low, not high. It helps generate heat to raise body temperature. During a fever above 41°C, shivering has already stopped - the issue is enzyme malfunction, not muscle damage.
C. Correct: Enzymes in the human body work best at an optimum temperature, around 37°C. When body temperature rises above 41°C, enzymes become overactive - their kinetic energy increases, leading to disruption of hydrogen bonds that maintain the enzyme’s 3D shape. As a result, enzymes begin to denature - their active sites change shape, and they can no longer catalyze vital metabolic reactions properly. This enzyme malfunction affects crucial processes such as cellular respiration, protein synthesis, and DNA replication, leading to cell and tissue damage. Therefore, a high fever is dangerous because overactive and denatured enzymes disrupt normal metabolism, potentially leading to organ failure or death.
D. Incorrect: Fever is actually part of the body’s defense mechanism to limit infection by inhibiting pathogen growth. A higher temperature does not cause infection to spread - instead, it can impair body function because of enzyme denaturation.
Question 8
Some bacteria can synthesize the amino acid isoleucine from threonine, a process involving five enzymes (E1 to E5) and four intermediary products (P, Q, R and S). The production of isoleucine is controlled by end-product inhibition.

Which statement describes this end-product inhibition?
A. If isoleucine accumulates, it inhibits the production of P.
B. End-product inhibition causes a build-up of intermediary products.
C. Isoleucine inhibits E5, so no more isoleucine is produced.
D. Isoleucine affects the structure of threonine.
Answer: A
A. Correct: When isoleucine (the final product) builds up to a high concentration, it binds to the allosteric site of the first enzyme (E1). This changes E1’s shape, preventing it from converting threonine 🡪 P. As a result, no more intermediates (P, Q, R, S) are made - the whole pathway slows down. When isoleucine levels drop, inhibition is removed, and E1 becomes active again. Therefore, if isoleucine accumulates, it inhibits the production of P.
B. Incorrect: If the inhibition happens at the first enzyme (E1). That means no intermediates (P, Q, R, or S) are formed - they actually decrease, not build up. A build-up would occur if a later enzyme were inhibited, but here it’s the first one.
C. Incorrect: The E5 is the last enzyme in the pathway. If E5 were inhibited, S (the second-last compound) would accumulate, but E1-E4 would still be active. In end-product inhibition, the end product inhibits the first enzyme (E1), not the last.
D. Incorrect: Isoleucine doesn’t react with threonine chemically. It binds only to the enzyme’s allosteric site, altering the enzyme’s shape, not the substrate’s structure. Threonine remains unchanged.
Question 9
Explain how temperature affects enzymes. [4]
Any four of the following:
a. speed of reaction/catalysis increases as temperature rises;
b. faster molecular motion so more collisions between substrate and active site;
c. denaturation at higher temperatures;
d. (denaturation causes) shape/conformation/structure of enzyme/active site altered/damaged;
e. an enzyme works fastest at its optimum temperature;
f. inactivation at lower temperatures (due to very few collisions);
g. sketch graph to model the effect of temperature on enzyme activity;
Sample answer:
As temperature increases, the rate of enzyme-catalyzed reactions also increases because higher temperatures cause molecules to move faster, leading to more frequent collisions between the substrate and the enzyme’s active site [2]. This results in a faster rate of catalysis up to an optimum temperature, where the enzyme works at its maximum efficiency [1]. However, if the temperature continues to rise beyond this optimum point, the enzyme begins to denature - its structure, especially the shape of the active site, becomes altered or damaged [2]. Once denatured, the enzyme can no longer bind to its substrate effectively, and the reaction rate drops sharply. At very low temperatures, enzyme activity is also reduced because molecular movement is slow, causing fewer successful collisions between enzymes and substrates [1].
Question 10
Outline the action of enzymes. [4]
Any four of the following:
a. catalyse/speed up «biological» reactions;
b. are substrate-specific;
c. lower the activation energy «of a chemical reaction»/makes reaction go more easily/increases likelihood of reaction happening;
d. substrate collides with/binds to active site;
e. enzyme–substrate complex/transition state formed OR bonds in substrate weakened;
Sample answer:
Enzymes catalyse or speed up biological reactions by lowering the activation energy needed for the reaction to occur [1].
Each enzyme is substrate-specific [1].
When the substrate collides with and binds to the enzyme’s active site, an enzyme–substrate complex is formed [1].
The enzyme helps weaken the bonds in the substrate, making it easier for the reaction to take place and form the product [1].
Question 11
Explain the regulation of metabolic pathways by end-product inhibition. [4]
Any four of the following:
a. final product in pathway acts as an inhibitor/blocks (reaction)/slows (reaction);
b. first/early/earlier enzyme (in pathway is inhibited);
c. non-competitive / binds at allosteric site / causes active site to change;
d. production of end-product reduced/paused when there is an excess;
e. isoleucine inhibits enzyme using threonine as substrate at start of pathway to isoleucine;
f. negative feedback / production restarts when end-product used up/concentration drops;
Sample answer:
End-product inhibition is a feedback control mechanism that prevents the overproduction of substances in metabolic pathways. In this process, the final product of a pathway acts as a non-competitive inhibitor of an enzyme that catalyzes an early step, usually the first one [2]. It binds to the enzyme’s allosteric site, changing the shape of the active site so that the substrate can no longer bind effectively, which slows or stops the reaction [2]. For example, in the pathway that converts threonine to isoleucine in bacteria, isoleucine acts as an inhibitor of the enzyme threonine deaminase, which catalyzes the first step. When isoleucine accumulates to high levels, it binds to threonine deaminase, preventing more isoleucine from being produced [1]. Once isoleucine levels drop because it is used in protein synthesis, inhibition is lifted, and the pathway resumes. This reversible feedback mechanism maintains metabolic balance and prevents wasteful overproduction of end-products.
Question 1
Cell metabolism involves anabolic and catabolic reactions. Which process directly involves anabolism?
A. Active transport of ions.
B. Release of energy from glucose.
C. Production of intracellular enzymes.
D. Breakdown of worn-out cell organelles by lysosomes.
Question 2
The graph shows the effect of temperature on the rate of a chemical reaction catalysed by enzymes.

What is a valid statement about a labelled point in the graph?
A. At P, substrate concentration is limiting the rate of reaction.
B. At Q, substrate and enzyme molecules achieve their highest kinetic energy.
C. At R, some active sites have changed shape.
D. At S, all substrate molecules have formed product.
Question 3
The diagram shows the formation of an enzyme–substrate complex.

What describes the process shown in the diagram?
A. The lock and key hypothesis, as the substrate is complementary to the enzyme.
B. The substrate permanently alters the shape of the enzyme’s active site.
C. The substrate and the active site have the same shape.
D. The active site changes shape to accommodate the substrate in the induced-fit model.
Question 4
Which statement applies to enzymes?
A. Enzyme function depends on collisions between substrate and active sites.
B. One active site typically binds to a broad range of substrates.
C. The active site on the substrate is specific to one enzyme.
D. When enzymes are immobilized they stop working.
Question 5
What effect do changes in pH have on enzymes?
A. All enzymes increase in activity as pH increases.
B. The activity of all enzymes is reduced by a pH below or above 7.
C. Low pH causes reversible denaturation in all enzymes.
D. Extreme pH can alter the active site of all enzymes.
Question 6
Which products are formed by the action of the enzymes protease and amylase?
| Protease | Amylase | |
| A. | fatty acids | glucose |
| B. | glycerol | fatty acids |
| C. | proteins | starch |
| D. | amino acids | maltose |
Question 7
A fever in a normally healthy adult during an illness is not usually a problem and can be regarded as a defence mechanism. However, a fever higher than 41°C might be dangerous. What is the cause of the possible damage due to a high fever?
A. Loss of body mass
B. Muscle damage due to shivering
C. Overactive metabolic enzymes
D. Spread of infection
Question 8
Some bacteria can synthesize the amino acid isoleucine from threonine, a process involving five enzymes (E1 to E5) and four intermediary products (P, Q, R and S). The production of isoleucine is controlled by end-product inhibition.

Which statement describes this end-product inhibition?
A. If isoleucine accumulates, it inhibits the production of P.
B. End-product inhibition causes a build-up of intermediary products.
C. Isoleucine inhibits E5, so no more isoleucine is produced.
D. Isoleucine affects the structure of threonine.
Question 9
Explain how temperature affects enzymes. [4]
Question 10
Outline the action of enzymes. [4]
Question 11
Explain the regulation of metabolic pathways by end-product inhibition. [4]