Question 1
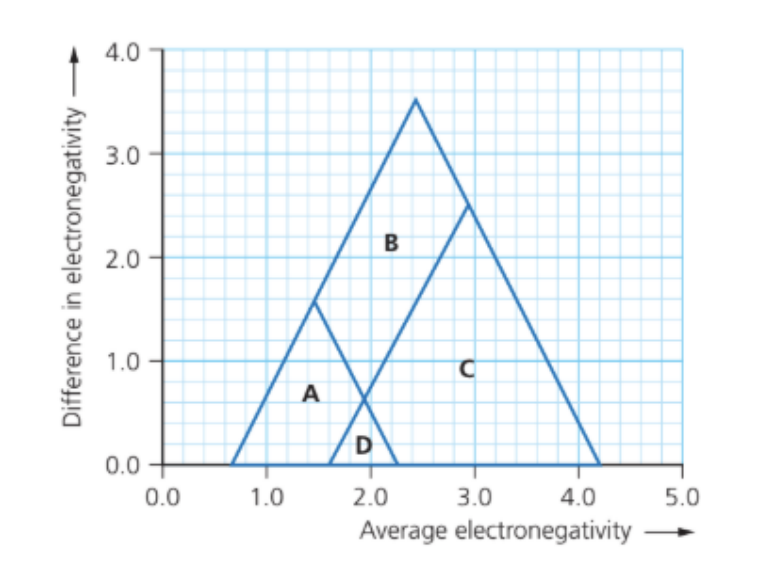
The electronegativity of antimony is 2.0 and that of chlorine is 3.2.
In which region of the bonding triangle diagram would SbCl5 be found?
Medium
Mark as Complete
Mark Scheme
Question 2
Two elements that have very similar electronegativity values chemically combine. The compound they form is plotted on a bonding triangle diagram. Which statement about the compound must be correct?
A. The compound has a low conductivity compared to a metal.
B. The covalent character is high.
C. The ionic character is high.
D. The metallic character is low.
Easy
Mark as Complete
Mark Scheme
Question 3
Which is the correct composition of the alloy stainless steel?
A. Iron, carbon and silver.
B. Iron, carbon and lead.
C. Iron, carbon and chromium.
D. Iron and carbon only.
Easy
Mark as Complete
Mark Scheme
Question 4
Which statement about polymers is correct?
A. All addition polymers are biodegradable.
B. All condensation polymers can be hydrolysed to release amino acids.
C. The formation of all addition polymers produces only one product.
D. All condensation polymers are naturally occurring.
Easy
Mark as Complete
Mark Scheme
Question 5
The diagram shows three repeat units in the structure of an addition polymer.
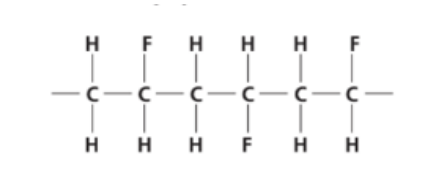
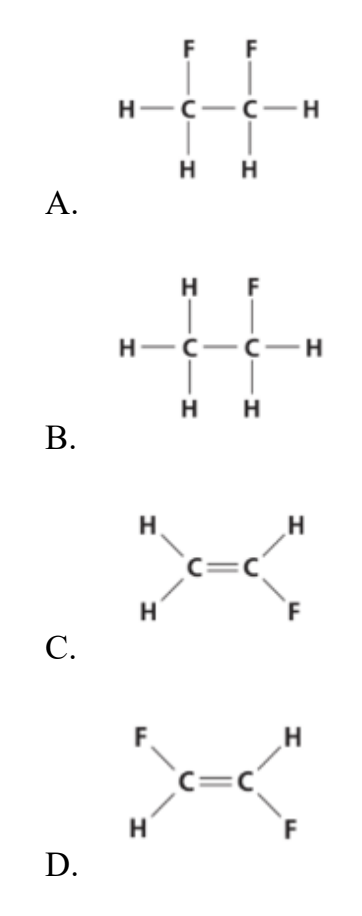
Which alkene monomer is used to make this polymer?
Easy
Mark as Complete
Mark Scheme
Question 6
In which row are the monomer and polymer chain correctly matched?
| Monomer | Polymer | |
| A. | CH3CH=CHCH3 | –CH(CH3)–CH(CH3)–CH(CH3)–CH(CH3)– |
| B. | CH2=CHF | –CH2–CHF–CHF–CHF– |
| C. | CH3CH=CH2 | –CH3–CH–CH2–CH3–CH–CH2– |
| D. | CH2=CHCH2CH3 | –CH2–CH2–CH₂–CH(CH2CH3)– |
Easy
Mark as Complete
Mark Scheme
Question 7
a. Define the term electronegativity.
b. Explain why elements with low electronegativities can conduct electricity.
c.
i. Explain, in terms of electronegativity, why ionic bonding results from the transfer of one or more electrons from one atom to another.
ii. Explain, in terms of electronegativities and ionization energy, why this transfer takes place.
Medium
Mark as Complete
Mark Scheme
Question 8
The following substances are all hard materials used in body armour: AlN, B₄C, SiC, Si₃N₄, and WC.
a. Plot labelled points for each compound on the bonding triangle diagram and deduce the type of bonding they are predicted to exhibit.
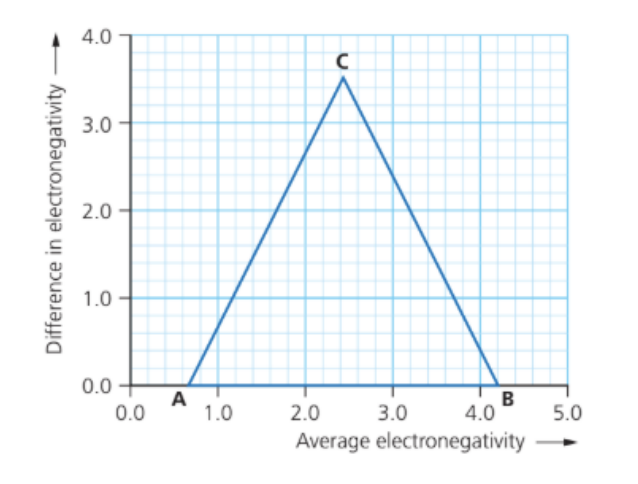
b. Compare the degree of metallic bonding in silicon carbide (SiC) with silicon nitride (Si₃N₄).
c. Suggest one limitation of using the bonding triangle diagram to predict bonding type
Medium
Mark as Complete
Mark Scheme
Question 9
Which row in the table describes the formation of a polymer?
| Monomer | Polymer | |
| A. | Ethane | Poly(ethane) |
| B. | Ethene | Poly(ethene) |
| C. | Ethane | Poly(ethene) |
| D. | Ethene | Poly(ethane) |
Easy
Mark as Complete
Mark Scheme
Question 10
Describe how, in general, alloying improves the usefulness of metals and how strength is increased in terms of structure. Use diagrams in your answers. No specific alloys need to be mentioned.
Medium
Mark as Complete
Mark Scheme
Question 11
State the name of the linkage present in polylactic acid whose repeating unit is shown below.
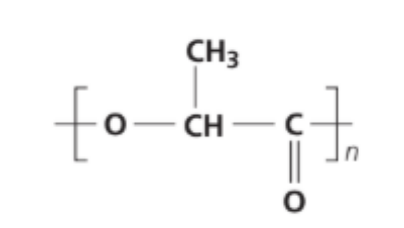
A. Ester.
B. Amide.
C. Peptide.
D. Carbonyl.
Easy
Mark as Complete
Mark Scheme
Question 12
The equation below shows the synthesis of Kevlar.
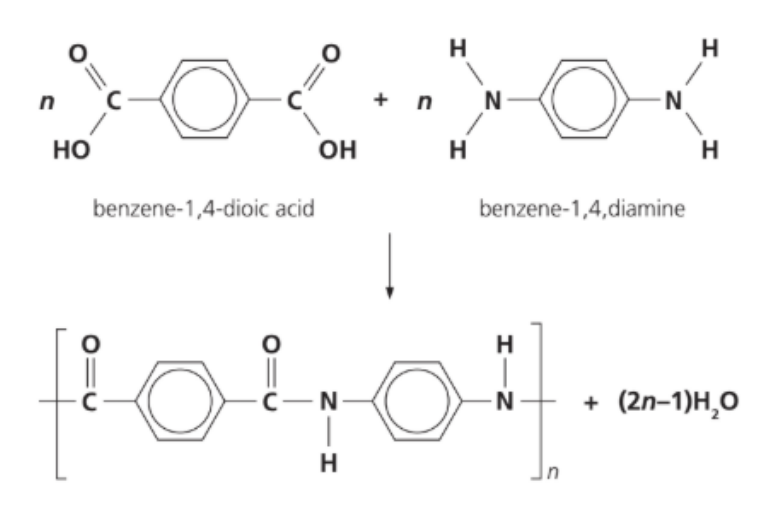
Which row describes Kevlar?
| Type of polymerization | Type of polymer | |
| A. | Condensation polymerization | Polyester |
| B. | Condensation polymerization | Polyamide |
| C. | Addition polymerization | Polyester |
| D. | Addition polymerization | Polyamide |
Medium
Mark as Complete
Mark Scheme
Question 13
Nylon-6,6 is a condensation polymer synthesized from hexanedioic acid and 1,6- diamino hexane. What type of linkage is present?
A. Carboxyl.
B. Amine.
C. Ester.
D. Amide.
Easy
Mark as Complete
Mark Scheme
Question 1
The electronegativity of antimony is 2.0 and that of chlorine is 3.2.
In which region of the bonding triangle diagram would SbCl5 be found?

Answer: Region C
ENagv = `frac{2.0+3.2}{2}`= 2.6
ΔEN =∣ 3.2 − 2.0 ∣= 1.2
Locate on the bonding triangle
• x-axis (average EN) → 2.6
• y-axis (difference in EN) → 1.2
This point lies roughly in region C of the diagram.
Question 2
Two elements that have very similar electronegativity values chemically combine. The compound they form is plotted on a bonding triangle diagram. Which statement about the compound must be correct?
A. The compound has a low conductivity compared to a metal.
B. The covalent character is high.
C. The ionic character is high.
D. The metallic character is low.
Answer: B. The covalent character is high.
A. Incorrect:
This statement is likely true for a covalent compound, as covalent compounds generally do not conduct electricity well in the solid or molten state. However, it is not the most direct or certain conclusion from the initial premise. Some covalent network solids (like graphite) can have high conductivity.
B. Correct:
The premise states that the two elements have "very similar electronegativity values." The difference in electronegativity (ΔEN) between two atoms is the primary factor in determining bond type. A small ΔEN means the electrons are shared relatively equally, which is the definition of a covalent bond. Therefore, the covalent character of the bond is high.
C. Incorrect:
Ionic bonds form when there is a large difference in electronegativity between the two atoms, causing one atom to essentially donate an electron to the other. Since the problem states the electronegativity values are "very similar," the ionic character is low, not high.
D. Incorrect:
Metallic character is a property of a single element (or a mixture of metals, forming an alloy). It is not a characteristic of a compound formed between two elements. The problem describes a compound, not a metallic element or alloy.
Question 3
Which is the correct composition of the alloy stainless steel?
A. Iron, carbon and silver.
B. Iron, carbon and lead.
C. Iron, carbon and chromium.
D. Iron and carbon only.
Answer: C. Iron, carbon and chromium.
Stainless steel is an alloy (a mixture of metals) designed to resist rusting and corrosion. It is mainly composed of:
• Iron (Fe) – the base metal
• Carbon (C) – increases strength and hardness
• Chromium (Cr) – provides corrosion resistance by forming a protective layer of chromium oxide (Cr₂O₃) on the surface
Question 4
Which statement about polymers is correct?
A. All addition polymers are biodegradable.
B. All condensation polymers can be hydrolysed to release amino acids.
C. The formation of all addition polymers produces only one product.
D. All condensation polymers are naturally occurring.
Answer: C. The formation of all addition polymers produces only one product.
A. Incorrect:
Most addition polymers (like polyethylene, polystyrene, PVC) are not biodegradable – they have strong C–C backbones that resist breakdown.
B. Incorrect:
Only polyamides (like proteins or nylon) yield amino acids on hydrolysis. Other condensation polymers like polyesters yield alcohols and carboxylic acids, not amino acids.
C. Correct:
In addition polymerization, no small molecule (like H₂O or HCl) is eliminated – only the polymer is formed.
D. Incorrect:
Some condensation polymers (like nylon, Terylene) are synthetic, while others (like proteins, cellulose) are natural. Not all are naturally occurring.
Question 5
The diagram shows three repeat units in the structure of an addition polymer.

Which alkene monomer is used to make this polymer?

Answer: D.
1. Identify the polymer’s repeating unit
From the diagram, each carbon chain unit looks like:
−CH2 − CHF −
That means the repeating unit has:
o One carbon bonded to two hydrogens (CH₂)
o The next carbon bonded to one hydrogen and one fluorine (CHF)
2. Reverse to the monomer
To form this polymer, the monomer must be an alkene (double bond between the two carbons).
Therefore, replace the single C–C bond with a double bond:
CH2 = CHF
Question 6
In which row are the monomer and polymer chain correctly matched?
| Monomer | Polymer | |
| A. | CH3CH=CHCH3 | –CH(CH3)–CH(CH3)–CH(CH3)–CH(CH3)– |
| B. | CH2=CHF | –CH2–CHF–CHF–CHF– |
| C. | CH3CH=CH2 | –CH3–CH–CH2–CH3–CH–CH2– |
| D. | CH2=CHCH2CH3 | –CH2–CH2–CH₂–CH(CH2CH3)– |
Answer: A. Monomer: CH3CH=CHCH3, Polymer: –CH(CH3)–CH(CH3)–CH(CH3)–CH(CH3)–
A. Correct:
Polymerizing but-2-ene gives a backbone where each carbon bears a CH3 substituent: –CH(CH3)–CH(CH3)–
B. Incorrect:
CH2=CHF should give –CH2–CHF–..., not –CHC–CHF–...
C. Incorrect:
CH3CH=CH2 (propene) should give –CH2–CH(CH3)–...; the shown chain places extra –CH3– units in the backbone.
D. Incorrect:
CH2=CHCH2CH3 (1-butene) should give –CH2–CH(CH2CH3)–..., not –CH2–CH2CH2–CH(CH2CH3)–...
Question 7
a. Define the term electronegativity.
b. Explain why elements with low electronegativities can conduct electricity.
c.
i. Explain, in terms of electronegativity, why ionic bonding results from the transfer of one or more electrons from one atom to another.
ii. Explain, in terms of electronegativities and ionization energy, why this transfer takes place.
a. Define the term electronegativity.
Electronegativity is the ability of an atom to attract the shared pair of electrons in a covalent bond toward itself.
b. Explain why elements with low electronegativities can conduct electricity. Elements with low electronegativities (typically metals) lose their valence electrons easily, forming a lattice of positive ions surrounded by delocalized electrons. These free-moving electrons allow the metal to conduct electricity. c.
i. Explain in terms of electronegativity why ionic bonding results from the transfer of one or more electrons from one atom to another.
Ionic bonding occurs because there is a large difference in electronegativity between two atoms.
The more electronegative atom attracts electrons away from the less electronegative atom, resulting in electron transfer and formation of ions.
ii. Explain in terms of electronegativities and ionization energy why this transfer takes place.
The atom with low electronegativity and low ionization energy loses electrons easily, while the atom with high electronegativity gains electrons readily. This makes electron transfer energetically favorable.
Question 8
The following substances are all hard materials used in body armour: AlN, B₄C, SiC, Si₃N₄, and WC.
a. Plot labelled points for each compound on the bonding triangle diagram and deduce the type of bonding they are predicted to exhibit.

b. Compare the degree of metallic bonding in silicon carbide (SiC) with silicon nitride (Si₃N₄).
c. Suggest one limitation of using the bonding triangle diagram to predict bonding type
a. Plot & bonding type
Use Pauling electronegativities:
Al 1.6, B 2.0, C 2.55, Si 1.9, N 3.0, W 2.36.
For each binary pair (X–Y):
Average EN = `frac{chi_X+chi_Y}{2}`, ΔEN = ∣ `""chi_X - chi_Y` ∣
| Compound | ENavg | ΔEN | Predicted region / bonding |
| AlN | ≈ 2.32 | ≈ 1.4 | High ionic / very polar-covalent (near the top of the triangle) |
| Si₃N₄ (Si–N) | ≈ 2.47 | ≈ 1.1 | Polar covalent |
| SiC | ≈ 2.23 | ≈ 0.65 | Covalent (network; slight polarity) |
| B₄C (B–C) | ≈ 2.30 | ≈ 0.5 | Covalent (network) |
| WC | ≈ 2.46 | ≈ 0.2 | Covalent–metallic border (mainly covalent with some metallic character) |
(When plotted, these points fall from near the covalent bottom edge (SiC, B₄C, WC) up toward the ionic apex (AlN), with Si₃N₄ in between.)
b. Compare metallic bonding in SiC vs Si₃N₄
SiC shows slightly more metallic character (smaller ΔEN, more delocalization / semiconducting).
Si₃N₄ is less metallic / more ionic-polar covalent (larger ΔEN), so it is a better insulator. c. One limitation of the bonding triangle
It uses only average EN and ΔEN, so it ignores structure and multi-center bonding (e.g., giant networks, delocalization, coordination/oxidation state). Hence, real compounds often have mixed bonding not captured by a single point.
Question 9
Which row in the table describes the formation of a polymer?
| Monomer | Polymer | |
| A. | Ethane | Poly(ethane) |
| B. | Ethene | Poly(ethene) |
| C. | Ethane | Poly(ethene) |
| D. | Ethene | Poly(ethane) |
Answer: B. Monomer: Ethene; Polymer: poly(ethene).
A. Incorrect: Ethane (C₂H₆) is saturated – no double bond → can’t polymerize.
B. Correct: Ethene (C₂H₄) is an alkene with a C=C double bond; under polymerization, the double bond opens to form long chains.
C. Incorrect: Monomer and polymer names don’t correspond.
D. Incorrect: Wrong polymer name — should be poly(ethene), not poly(ethane).
Question 10
Describe how, in general, alloying improves the usefulness of metals and how strength is increased in terms of structure. Use diagrams in your answers. No specific alloys need to be mentioned.
1. Structure of pure metals (diagram 1)
• In a pure metal, atoms are arranged in layers of identical atoms in a regular, close packed lattice.
• These layers of atoms can slide easily over each other when a force is applied → Metals are malleable and ductile.
2. Structure of an alloy (diagram 2)
• When another element (metal or non-metal) is added, the atoms of the added element have a different size.
• This distorts the regular layers in the metal lattice, making it harder for the layers to slide over each other.
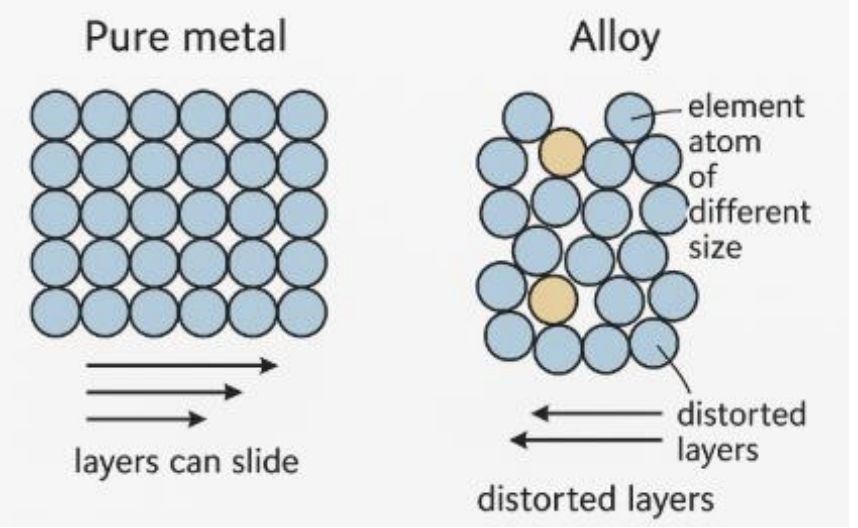
3. Explanation of increased strength
• The distortion caused by atoms of different sizes prevents dislocation movement in the lattice.
• Hence, alloys are harder and stronger than pure metals.
• They are also less malleable and less ductile because atoms cannot easily slide. 4. Improved usefulness
Alloying allows metals to have enhanced properties such as:
• Greater strength and hardness
• Resistance to corrosion
• Improved durability and tensile strength
• Better suitability for specific applications (e.g., construction, tools, machinery)
Question 11
State the name of the linkage present in polylactic acid whose repeating unit is shown below.

A. Ester.
B. Amide.
C. Peptide.
D. Carbonyl.
Answer: A. Ester
The repeating unit: –O–CH(CH₃)–C(=O)–
This segment contains a –COO– linkage, which is characteristic of an ester functional group.
A. Correct: Present between the lactic acid monomers after condensation. This is what forms the polymer chain in polylactic acid (PLA).
B. Incorrect: Requires nitrogen; no N atom here.
C. Incorrect: A specific type of amide linkage found in proteins, not in PLA.
D. Incorrect: A carbonyl group is part of the structure but not the linking group between monomers.
Question 12
The equation below shows the synthesis of Kevlar.

Which row describes Kevlar?
| Type of polymerization | Type of polymer | |
| A. | Condensation polymerization | Polyester |
| B. | Condensation polymerization | Polyamide |
| C. | Addition polymerization | Polyester |
| D. | Addition polymerization | Polyamide |
Answer: B. Condensation polymerization; Polyamide.
• Type of polymerization:
A small molecule (H₂O) is eliminated during each linkage formation → This is condensation polymerization. It is not addition polymerization because no double bonds are involved.
• Type of polymer:
The linkage formed is –CONH–, called an amide (peptide) linkage. Therefore, Kevlar is a polyamide (similar to nylon).
Question 13
Nylon-6,6 is a condensation polymer synthesized from hexanedioic acid and 1,6- diamino hexane. What type of linkage is present?
A. Carboxyl.
B. Amine.
C. Ester.
D. Amide.
Answer: D. Amide.
Nylon-6,6 is made from:
• Hexanedioic acid (adipic acid) — contains –COOH groups
• 1,6-diaminohexane (hexamethylenediamine) — contains –NH₂ groups When these react together, the –COOH and –NH₂ groups combine to form –CONH– linkages and release water (H₂O). This is the characteristic linkage of a polyamide.
Question 1
The electronegativity of antimony is 2.0 and that of chlorine is 3.2.
In which region of the bonding triangle diagram would SbCl5 be found?

Question 2
Two elements that have very similar electronegativity values chemically combine. The compound they form is plotted on a bonding triangle diagram. Which statement about the compound must be correct?
A. The compound has a low conductivity compared to a metal.
B. The covalent character is high.
C. The ionic character is high.
D. The metallic character is low.
Question 3
Which is the correct composition of the alloy stainless steel?
A. Iron, carbon and silver.
B. Iron, carbon and lead.
C. Iron, carbon and chromium.
D. Iron and carbon only.
Question 4
Which statement about polymers is correct?
A. All addition polymers are biodegradable.
B. All condensation polymers can be hydrolysed to release amino acids.
C. The formation of all addition polymers produces only one product.
D. All condensation polymers are naturally occurring.
Question 5
The diagram shows three repeat units in the structure of an addition polymer.

Which alkene monomer is used to make this polymer?

Question 6
In which row are the monomer and polymer chain correctly matched?
| Monomer | Polymer | |
| A. | CH3CH=CHCH3 | –CH(CH3)–CH(CH3)–CH(CH3)–CH(CH3)– |
| B. | CH2=CHF | –CH2–CHF–CHF–CHF– |
| C. | CH3CH=CH2 | –CH3–CH–CH2–CH3–CH–CH2– |
| D. | CH2=CHCH2CH3 | –CH2–CH2–CH₂–CH(CH2CH3)– |
Question 7
a. Define the term electronegativity.
b. Explain why elements with low electronegativities can conduct electricity.
c.
i. Explain, in terms of electronegativity, why ionic bonding results from the transfer of one or more electrons from one atom to another.
ii. Explain, in terms of electronegativities and ionization energy, why this transfer takes place.
Question 8
The following substances are all hard materials used in body armour: AlN, B₄C, SiC, Si₃N₄, and WC.
a. Plot labelled points for each compound on the bonding triangle diagram and deduce the type of bonding they are predicted to exhibit.

b. Compare the degree of metallic bonding in silicon carbide (SiC) with silicon nitride (Si₃N₄).
c. Suggest one limitation of using the bonding triangle diagram to predict bonding type
Question 9
Which row in the table describes the formation of a polymer?
| Monomer | Polymer | |
| A. | Ethane | Poly(ethane) |
| B. | Ethene | Poly(ethene) |
| C. | Ethane | Poly(ethene) |
| D. | Ethene | Poly(ethane) |
Question 10
Describe how, in general, alloying improves the usefulness of metals and how strength is increased in terms of structure. Use diagrams in your answers. No specific alloys need to be mentioned.
Question 11
State the name of the linkage present in polylactic acid whose repeating unit is shown below.

A. Ester.
B. Amide.
C. Peptide.
D. Carbonyl.
Question 12
The equation below shows the synthesis of Kevlar.

Which row describes Kevlar?
| Type of polymerization | Type of polymer | |
| A. | Condensation polymerization | Polyester |
| B. | Condensation polymerization | Polyamide |
| C. | Addition polymerization | Polyester |
| D. | Addition polymerization | Polyamide |
Question 13
Nylon-6,6 is a condensation polymer synthesized from hexanedioic acid and 1,6- diamino hexane. What type of linkage is present?
A. Carboxyl.
B. Amine.
C. Ester.
D. Amide.