Question 1
A temperature change of 6.4°C is measured when adding 5.0 cm3 of 1.0 mol dm-3 hydrochloric acid to 5.0 cm3 of 1.0 mol dm-3 potassium hydroxide. Both solutions had the same initial temperature. For the resulting mixture assume the density is 1.0 g cm-3 and the specific heat capacity is 4.18 J g-1 K-1.
What is the change in enthalpy of the reaction in kJ mol-1?
A. ΔH° = `- frac{10*4.18*6.4}{1.0*0.005}`
B. ΔH° = `- frac{10*4.18*6.4}{1.0*0.005*2.0}`
C. ΔH° = `- frac{10*4.18*6.4}{1.0*0.005*1000}`
D. ΔH° = `- frac{10*4.18*6.4}{10*0.005*2*1000}`
Medium
Mark as Complete
Mark Scheme
Question 2
The potential energy profile of a reaction is shown.

What can be determined about stability and energy change from the potential energy profile shown?
| More stable | Reaction | |
| A. | Reactants | Exothermic |
| B. | Reactants | Endothermic |
| C. | Products | Exothermic |
| D. | Products | Endothermic |
Medium
Mark as Complete
Mark Scheme
Question 3
When 25.0 cm³ 0.100 mol dm-3 NaOH (aq) is mixed with 25.0 cm³ 0.100 mol dm-3 HCl (aq) at the same temperature, a temperature rise, ΔT, is recorded. What is the expression, in kJ mol-1, for the enthalpy of neutralization?
(Assume the density of the mixture = 1.00 g cm⁻³ and its specific heat capacity = 4.18 kJ kg-1 K-1.)
A. `-frac{25.0*4.18*ΔT}{50.0*0.100}`
B. `-frac{25.0*4.18*ΔT}{25.0*0.100}`
C. `-frac{50.0*4.18*ΔT}{50.0*0.100}`
D. `-frac{50.0*4.18*ΔT}{25.0*0.100}`
Medium
Mark as Complete
Mark Scheme
Question 4
The data below is from an experiment used to measure the enthalpy change for the combustion of sucrose (common table sugar), C₁₂H₂₂O₁₁(s). The time–temperature data was taken from a data-logging software program.
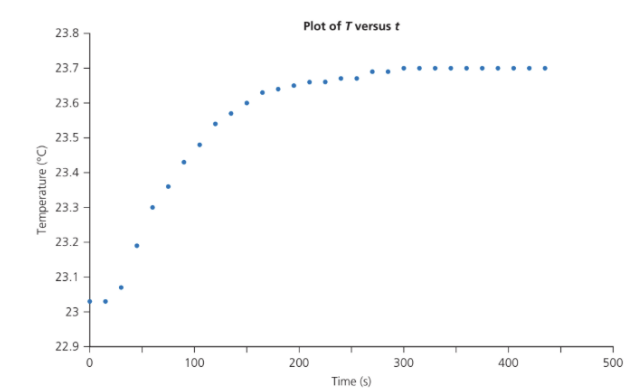
Mass of sample of sucrose, m = 0.4835 g.
Heat capacity of the system, Csystem = 10.114 kJ K-1.
a. Calculate ΔT for the water surrounding the chamber in the calorimeter. b. Determine the amount, in moles, of sucrose.
c.
i) Calculate the enthalpy change for the combustion of 1 mole of sucrose.
ii) The literature value for the combustion of sucrose is –5.6 × 10³ kJ mol-1. Calculate the percentage error in your calculated value.
Hard
Mark as Complete
Mark Scheme
Question 5
In aqueous solution, lithium hydroxide and hydrochloric acid react as follows.
LiOH (aq) + HCl (aq) → LiCl (aq) + H₂O (l)
The data below is from an experiment to determine the standard enthalpy change of this reaction.
50.0 cm3 of a 0.500 mol dm-3 solution of LiOH was mixed rapidly in a glass beaker with 50.0 cm3 of a 0.500 mol dm-3 solution of HCl.
Initial temperature of each solution = 20.6 °C
Final temperature of the mixture = 24.1 °C
a. State, with a reason, whether the reaction is exothermic or endothermic.
b. Explain why the solutions were mixed rapidly.
c. Calculate the enthalpy change of this reaction in kJ mol-1. Assume that the specific heat capacity of the solution is the same as that of water.
d. The experiment was repeated but with an HCl concentration of 0.520 mol dm-3 instead of 0.500 mol dm-3. State and explain what the temperature change would be.
Hard
Mark as Complete
Mark Scheme
Question 6
Which statement is correct for this reaction?
Fe₂O₃(s) + 3CO(g) → 2Fe(s) + 3CO₂(g) ΔH° = –26.6 kJ
A. 13.3 kJ are released for every mole of Fe produced.
B. 26.6 kJ are absorbed for every mole of Fe produced.
C. 53.2 kJ are released for every mole of Fe produced.
D. 26.6 kJ are released for every mole of Fe produced.
Medium
Mark as Complete
Mark Scheme
Question 7
What is the temperature rise when 2100 J of energy is supplied to 100 g of water? (Specific heat capacity of water = 4.2 J g-1 K-1.)
A. 5 °C.
B. 278 K.
C. 0.2 °C.
D. 20 °C.
Medium
Mark as Complete
Mark Scheme
Question 8
Consider the following two equations.
2Ca(s) + O₂(g) → 2CaO(s) ΔH° = +x kJ
Ca(s) + 0.5O₂(g) + CO₂(g) → CaCO₃(s) ΔH° = +y kJ
What is ΔH°, in kJ, for the following reaction?
CaO(s) + CO₂(g) → CaCO₃(s)
A. y − 0.5x
B. y – x
C. 0.5 – y
D. x – y
Medium
Mark as Complete
Mark Scheme
Question 9
The table shows the specific heat capacities of four metals.
| Metal | Specific heat capacity (J g⁻¹ K⁻¹) |
| Copper | 0.385 |
| Magnesium | 1.02 |
| Mercury | 0.138 |
| Lead | 0.129 |
If 100 kJ of heat energy is absorbed by 10.0 g samples of each of the metals above, which are all at 25°C, which metal will have the lowest temperature?
A. Copper.
B. Magnesium.
C. Mercury.
D. Lead.
Medium
Mark as Complete
Mark Scheme
Question 10
To determine the enthalpy change of combustion of methanol, CH₃OH, 0.230 g of methanol was combusted in a spirit burner. The heat released increased the temperature of 50.0 cm³ of water from 24.5°C to 45.8°C. Calculate the enthalpy change of combustion of methanol.
Medium
Mark as Complete
Mark Scheme
Question 1
A temperature change of 6.4°C is measured when adding 5.0 cm3 of 1.0 mol dm-3 hydrochloric acid to 5.0 cm3 of 1.0 mol dm-3 potassium hydroxide. Both solutions had the same initial temperature. For the resulting mixture assume the density is 1.0 g cm-3 and the specific heat capacity is 4.18 J g-1 K-1.
What is the change in enthalpy of the reaction in kJ mol-1?
A. ΔH° = `- frac{10*4.18*6.4}{1.0*0.005}`
B. ΔH° = `- frac{10*4.18*6.4}{1.0*0.005*2.0}`
C. ΔH° = `- frac{10*4.18*6.4}{1.0*0.005*1000}`
D. ΔH° = `- frac{10*4.18*6.4}{10*0.005*2*1000}`
Answer: C. ΔH° = `- frac{10*4.18*6.4}{1.0*0.005*1000}`
• Moles of HCl (and KOH)
n = 1.0 mol dm−3`*frac{5.0}{1000}`
• Enthalpy change required in kJ mol-1.
Step 1: Calculate heat released (q)
q = mcΔT = 10 × 4.18 × 6.4 = 267.52 J
Step 2: Convert to enthalpy change per mole
Reaction involves 0.005 mol of acid and base (1:1 stoichiometry).
ΔH = `frac{-q}[n}`
ΔH = `frac{-267.52}{0.005}`= −53504 J mol−1 = −53.5 kJ mol−1
Step 3: Match with given formulas
The correct formula must include:
• 10 × 4.18 × 6.4(heat in J)
• divided by 1.0 × 0.005 × 1000(convert to kJ mol-1)
That matches Option C: ΔH° = `- frac{10*4.18*6.4}{1.0*0.005*1000}`
Question 2
The potential energy profile of a reaction is shown.

What can be determined about stability and energy change from the potential energy profile shown?
| More stable | Reaction | |
| A. | Reactants | Exothermic |
| B. | Reactants | Endothermic |
| C. | Products | Exothermic |
| D. | Products | Endothermic |
Answer: C. More stable: Products; Reaction: Exothermic.
Step 1 – What the diagram shows
• The reactants start at a higher potential energy.
• The products are at a lower potential energy (the arrow points downward). → This means energy is released during the reaction.
Step 2 – Type of reaction
Step 3 – Stability
The species with lower potential energy is more stable. → Since the products are at lower energy, products are more stable.
Question 3
When 25.0 cm³ 0.100 mol dm-3 NaOH (aq) is mixed with 25.0 cm³ 0.100 mol dm-3 HCl (aq) at the same temperature, a temperature rise, ΔT, is recorded. What is the expression, in kJ mol-1, for the enthalpy of neutralization?
(Assume the density of the mixture = 1.00 g cm⁻³ and its specific heat capacity = 4.18 kJ kg-1 K-1.)
A. `-frac{25.0*4.18*ΔT}{50.0*0.100}`
B. `-frac{25.0*4.18*ΔT}{25.0*0.100}`
C. `-frac{50.0*4.18*ΔT}{50.0*0.100}`
D. `-frac{50.0*4.18*ΔT}{25.0*0.100}`
Answer: D. `-frac{50.0*4.18*ΔT}{25.0*0.100}`
• Heat released by the mixture (50.0 g = 0.0500 kg)
q = mcΔT = 0.0500 kg × 4.18 kJ kg-1 K-1 × ΔT
• Moles reacting (and H₂O formed):
n = 0.100 mol dm-3`*frac{"25.0""cm^3}{1000}`= 0.00250 mol
ΔH = `frac{-q}{n}`= `-frac{50.0*4.18*ΔT}{25.0*0.100}`
Question 4
The data below is from an experiment used to measure the enthalpy change for the combustion of sucrose (common table sugar), C₁₂H₂₂O₁₁(s). The time–temperature data was taken from a data-logging software program.

Mass of sample of sucrose, m = 0.4835 g.
Heat capacity of the system, Csystem = 10.114 kJ K-1.
a. Calculate ΔT for the water surrounding the chamber in the calorimeter. b. Determine the amount, in moles, of sucrose.
c.
i) Calculate the enthalpy change for the combustion of 1 mole of sucrose.
ii) The literature value for the combustion of sucrose is –5.6 × 10³ kJ mol-1. Calculate the percentage error in your calculated value.
a. ΔT
Initial ≈ 23.0 °C → ΔT = 23.7 − 23.0 = 0.7 K
b. Moles of sucrose
Mᵣ(sucrose) ≈ 342.3
→ n = `frac{0.4835}{342.3}`= 1.41 × 10-3 mol
c.
i. ΔH (per mole)
q = CsystemΔT = 10.114 × 0.7 = 7.0798 kJ
ΔH = `frac{-q}{n} = frac{−7.0798}{1.41*10^3}` ≈ − 5.01 × 10³ kJ mol-1
ii. % error vs literature (−5.6 × 10³ kJ mol-1)
% error = `frac{|−5.01 − (−5.60)|}{5.60*100}` ≈ 10.5%
Question 5
In aqueous solution, lithium hydroxide and hydrochloric acid react as follows.
LiOH (aq) + HCl (aq) → LiCl (aq) + H₂O (l)
The data below is from an experiment to determine the standard enthalpy change of this reaction.
50.0 cm3 of a 0.500 mol dm-3 solution of LiOH was mixed rapidly in a glass beaker with 50.0 cm3 of a 0.500 mol dm-3 solution of HCl.
Initial temperature of each solution = 20.6 °C
Final temperature of the mixture = 24.1 °C
a. State, with a reason, whether the reaction is exothermic or endothermic.
b. Explain why the solutions were mixed rapidly.
c. Calculate the enthalpy change of this reaction in kJ mol-1. Assume that the specific heat capacity of the solution is the same as that of water.
d. The experiment was repeated but with an HCl concentration of 0.520 mol dm-3 instead of 0.500 mol dm-3. State and explain what the temperature change would be.
a. Exothermic. The temperature of the mixture rises from 20.6 °C to 24.1 °C (ΔT = +3.5 K), so heat is released to the surroundings.
b. To minimise heat loss (approximate an adiabatic measurement) and to ensure uniform mixing so the maximum temperature rise is captured accurately.
c. Enthalpy change, ΔH, in kJ mol-1
• Volumes: 50.0 cm³ + 50.0 cm³ → total mass ≈ 100.0 g (density ≈ 1.00 g cm⁻³).
• ΔT = 3.5 K; c = 4.18 J g-1 K-1.
Heat released:
q = mcΔT = 100.0 × 4.18 × 3.5 = 1.463 × 10³ J
Moles reacting (1:1):
n(LiOH) = 0.500 mol dm-3 × 0.0500 dm3 = 0.0250 mol
(same for HCl; complete neutralisation → 0.0250 mol H₂O formed)
Enthalpy change per mole:
ΔH = `frac{-q}{n}`= `frac{−1.463*103}{0.0250}` = − 5.85 × 10⁴ J mol-1 = − 58.5 kJ mol-1
(negative sign because the reaction is exothermic)
d. If HCl is 0.520 mol dm-3 instead of 0.500 mol dm-3, what happens to ΔT?
Moles now:
n(HCl) = 0.520 × 0.0500 = 0.0260 mol.
LiOH remains 0.0250 mol → LiOH is limiting, and 0.0250 mol still reacts. Since the heat released depends on moles neutralised (≈ 0.0250 mol, same as before) and the mass/heat capacity of the mixture is essentially unchanged, ΔT will be about the same (≈ 3.5 K). (Any difference would be negligible.)
Question 6
Which statement is correct for this reaction?
Fe₂O₃(s) + 3CO(g) → 2Fe(s) + 3CO₂(g) ΔH° = –26.6 kJ
A. 13.3 kJ are released for every mole of Fe produced.
B. 26.6 kJ are absorbed for every mole of Fe produced.
C. 53.2 kJ are released for every mole of Fe produced.
D. 26.6 kJ are released for every mole of Fe produced.
Answer: A. 13.3 kJ are released for every mole of Fe produced.
Step 1. What does the negative sign mean?
• A negative ΔH means the reaction is exothermic, i.e., energy is released. Step 2. How many moles of Fe are produced per reaction?
• The given equation shows 2 moles of Fe produced per reaction, while ΔH° = −26.6 kJ refers to the whole reaction as written.
Step 3. Energy released per mole of Fe
Energy released per mole of Fe = `frac{26.6}{2}`= 13.3 kJ mol-1
Question 7
What is the temperature rise when 2100 J of energy is supplied to 100 g of water? (Specific heat capacity of water = 4.2 J g-1 K-1.)
A. 5 °C.
B. 278 K.
C. 0.2 °C.
D. 20 °C.
Answer: A. 5 °C.
q = mcΔT
→ ΔT = `frac{q}{mc}`= `frac{2100}{100*4.2}`= `frac{2100}{420}`= 5.0
→ ΔT = 5.0
Question 8
Consider the following two equations.
2Ca(s) + O₂(g) → 2CaO(s) ΔH° = +x kJ
Ca(s) + 0.5O₂(g) + CO₂(g) → CaCO₃(s) ΔH° = +y kJ
What is ΔH°, in kJ, for the following reaction?
CaO(s) + CO₂(g) → CaCO₃(s)
A. y − 0.5x
B. y – x
C. 0.5 – y
D. x – y
Answer: A. y − 0.5x
• 2Ca(s) + O2(g) → 2CaO(s), ∆H∘ = +x kJ
Divide by 2:
Ca(s) + 0.5O2(g) → CaO(s), ΔH = `frac{+x}{2}`kJ (1)
• Ca(s) + 0.5O2(g) + CO2(g) → CaCO3(s), ∆H∘ = +y kJ (2)
We want:
CaO(s) + CO2(g) → CaCO3(s)
Now subtract equation (1) (reversed) from equation (2):
Ca(s) + 0.5O2(g) + CO2(g) → CaCO3(s)
minus
Ca(s) + 0.5O2(g) → CaO(s)
This gives:
CaO(s) + CO2(g) → CaCO3(s)
Combine enthalpy changes:
ΔH° = y − `frac{x}{2}`
Question 9
The table shows the specific heat capacities of four metals.
| Metal | Specific heat capacity (J g⁻¹ K⁻¹) |
| Copper | 0.385 |
| Magnesium | 1.02 |
| Mercury | 0.138 |
| Lead | 0.129 |
If 100 kJ of heat energy is absorbed by 10.0 g samples of each of the metals above, which are all at 25°C, which metal will have the lowest temperature?
A. Copper.
B. Magnesium.
C. Mercury.
D. Lead.
Answer: B. Magnesium.
Each 10.0 g metal sample absorbs the same amount of heat:
q = 100 kJ = 100,000 J
We use:
q = mcΔT ⇒ ΔT = `frac{q}{mc}`
Compare ΔT for each metal:
All have the same q and m, so:
ΔT = `frac{1}{prop}`
The smaller the specific heat capacity, the larger the temperature rise. Specific heat capacities
| Metal | c (J g-1 K-1) |
| Copper | 0.385 |
| Magnesium | 1.02 |
| Mercury | 0.138 |
| Lead | 0.129 |
Interpretation
• Largest c→ smallest ΔT → lowest final temperature.
• The largest c = 1.02 J g-1 K-1(magnesium).
Question 10
To determine the enthalpy change of combustion of methanol, CH₃OH, 0.230 g of methanol was combusted in a spirit burner. The heat released increased the temperature of 50.0 cm³ of water from 24.5°C to 45.8°C. Calculate the enthalpy change of combustion of methanol.
Step 1. Calculate heat absorbed by the water
q = mcΔT = 50.0 × 4.18 × 21.3 = 4450.5 J = 4.45 kJ
Since the reaction is exothermic, heat released by methanol = –4.45 kJ.
Step 2. Moles of methanol burned
Molar mass of CH3OH = 12.0 + (4 × 1.0) + 16.0 = 32.0 g mol-1
n = `frac{0.230}{32.0}`= 0.00719 mol
Step 3. Enthalpy change of combustion (per mole)
ΔH = `frac{−4.45}{0.00719}`= −619 kJ mol-1
Question 1
A temperature change of 6.4°C is measured when adding 5.0 cm3 of 1.0 mol dm-3 hydrochloric acid to 5.0 cm3 of 1.0 mol dm-3 potassium hydroxide. Both solutions had the same initial temperature. For the resulting mixture assume the density is 1.0 g cm-3 and the specific heat capacity is 4.18 J g-1 K-1.
What is the change in enthalpy of the reaction in kJ mol-1?
A. ΔH° = `- frac{10*4.18*6.4}{1.0*0.005}`
B. ΔH° = `- frac{10*4.18*6.4}{1.0*0.005*2.0}`
C. ΔH° = `- frac{10*4.18*6.4}{1.0*0.005*1000}`
D. ΔH° = `- frac{10*4.18*6.4}{10*0.005*2*1000}`
Question 2
The potential energy profile of a reaction is shown.

What can be determined about stability and energy change from the potential energy profile shown?
| More stable | Reaction | |
| A. | Reactants | Exothermic |
| B. | Reactants | Endothermic |
| C. | Products | Exothermic |
| D. | Products | Endothermic |
Question 3
When 25.0 cm³ 0.100 mol dm-3 NaOH (aq) is mixed with 25.0 cm³ 0.100 mol dm-3 HCl (aq) at the same temperature, a temperature rise, ΔT, is recorded. What is the expression, in kJ mol-1, for the enthalpy of neutralization?
(Assume the density of the mixture = 1.00 g cm⁻³ and its specific heat capacity = 4.18 kJ kg-1 K-1.)
A. `-frac{25.0*4.18*ΔT}{50.0*0.100}`
B. `-frac{25.0*4.18*ΔT}{25.0*0.100}`
C. `-frac{50.0*4.18*ΔT}{50.0*0.100}`
D. `-frac{50.0*4.18*ΔT}{25.0*0.100}`
Question 4
The data below is from an experiment used to measure the enthalpy change for the combustion of sucrose (common table sugar), C₁₂H₂₂O₁₁(s). The time–temperature data was taken from a data-logging software program.

Mass of sample of sucrose, m = 0.4835 g.
Heat capacity of the system, Csystem = 10.114 kJ K-1.
a. Calculate ΔT for the water surrounding the chamber in the calorimeter. b. Determine the amount, in moles, of sucrose.
c.
i) Calculate the enthalpy change for the combustion of 1 mole of sucrose.
ii) The literature value for the combustion of sucrose is –5.6 × 10³ kJ mol-1. Calculate the percentage error in your calculated value.
Question 5
In aqueous solution, lithium hydroxide and hydrochloric acid react as follows.
LiOH (aq) + HCl (aq) → LiCl (aq) + H₂O (l)
The data below is from an experiment to determine the standard enthalpy change of this reaction.
50.0 cm3 of a 0.500 mol dm-3 solution of LiOH was mixed rapidly in a glass beaker with 50.0 cm3 of a 0.500 mol dm-3 solution of HCl.
Initial temperature of each solution = 20.6 °C
Final temperature of the mixture = 24.1 °C
a. State, with a reason, whether the reaction is exothermic or endothermic.
b. Explain why the solutions were mixed rapidly.
c. Calculate the enthalpy change of this reaction in kJ mol-1. Assume that the specific heat capacity of the solution is the same as that of water.
d. The experiment was repeated but with an HCl concentration of 0.520 mol dm-3 instead of 0.500 mol dm-3. State and explain what the temperature change would be.
Question 6
Which statement is correct for this reaction?
Fe₂O₃(s) + 3CO(g) → 2Fe(s) + 3CO₂(g) ΔH° = –26.6 kJ
A. 13.3 kJ are released for every mole of Fe produced.
B. 26.6 kJ are absorbed for every mole of Fe produced.
C. 53.2 kJ are released for every mole of Fe produced.
D. 26.6 kJ are released for every mole of Fe produced.
Question 7
What is the temperature rise when 2100 J of energy is supplied to 100 g of water? (Specific heat capacity of water = 4.2 J g-1 K-1.)
A. 5 °C.
B. 278 K.
C. 0.2 °C.
D. 20 °C.
Question 8
Consider the following two equations.
2Ca(s) + O₂(g) → 2CaO(s) ΔH° = +x kJ
Ca(s) + 0.5O₂(g) + CO₂(g) → CaCO₃(s) ΔH° = +y kJ
What is ΔH°, in kJ, for the following reaction?
CaO(s) + CO₂(g) → CaCO₃(s)
A. y − 0.5x
B. y – x
C. 0.5 – y
D. x – y
Question 9
The table shows the specific heat capacities of four metals.
| Metal | Specific heat capacity (J g⁻¹ K⁻¹) |
| Copper | 0.385 |
| Magnesium | 1.02 |
| Mercury | 0.138 |
| Lead | 0.129 |
If 100 kJ of heat energy is absorbed by 10.0 g samples of each of the metals above, which are all at 25°C, which metal will have the lowest temperature?
A. Copper.
B. Magnesium.
C. Mercury.
D. Lead.
Question 10
To determine the enthalpy change of combustion of methanol, CH₃OH, 0.230 g of methanol was combusted in a spirit burner. The heat released increased the temperature of 50.0 cm³ of water from 24.5°C to 45.8°C. Calculate the enthalpy change of combustion of methanol.