Question 1
What is the order of increasing acidity?
| Acid | pKₐ | Acid | Kₐ |
| HClO | 7.4 | HF | 5.6 × 10⁻⁴ |
| HIO₃ | 0.8 | CH₃CH₂COOH | 1.3 × 10⁻⁵ |
A. HClO < CH₃CH₂COOH < HF < HIO₃
B. HClO < HF < CH₃CH₂COOH < HIO₃
C. HIO₃ < HF < CH₃CH₂COOH < HClO
D. HIO₃ < CH₃CH₂COOH< HF < HClO
Medium
Mark as Complete
Mark Scheme
Question 2
a. Explain why a 1.0 mol dm⁻3 solution of sodium hydroxide has a pH of 14 whereas 1.0 mol dm⁻3 ammonia solution has a pH of about 12. Use equations in your answer.
b. 20.0 cm3 of a known concentration of sodium hydroxide is titrated with a solution of nitric acid. The graph for this titration is given below.

i. State an equation for the reaction between sodium hydroxide and nitric acid.
ii. Calculate the concentration of the sodium hydroxide solution before the titration.
iii. From the graph determine the volume of nitric acid required to neutralize the sodium hydroxide and calculate the concentration of the nitric acid.
iv. Predict the volume of ethanoic acid of the same concentration as the nitric acid in (b)(iii), required to neutralize 20.0 cm3 of this sodium hydroxide solution.
Hard
Mark as Complete
Mark Scheme
Question 3
The graph below shows the change in pH when aqueous sodium hydroxide is added to 20 cm³ of aqueous hydrochloric acid.

By reference to the graph:
a. State the [H⁺] before any alkali is added.
b. State by how much the [H⁺] changes after the addition of 20 cm³ of aqueous sodium hydroxide.
c. Determine the volume of the same sodium hydroxide solution needed to neutralize 20 cm³ of aqueous ethanoic acid of the same concentration as the hydrochloric acid.
Medium
Mark as Complete
Mark Scheme
Question 4
Carbonic acid can be used to treat wasp (an insect) stings.
a. Suggest what this indicates about the nature of wasp stings.
b. Name the type of reaction that occurs.
c. Explain why hydrochloric acid is not used to treat wasp stings.
Medium
Mark as Complete
Mark Scheme
Question 5
Five unlabelled bottles are known to contain the following 0.10 mol dm⁻³ aqueous solutions:
CH₃COOH, NaCl, NaOH, HCl, NH₃
a. Describe and explain how the pH values of these five solutions could be used to identify them.
b. Experiments were conducted to illustrate some properties of sodium hydrogencarbonate, NaHCO₃.
i. In one experiment some solid NaHCO₃ was added to aqueous NaOH. After stirring the pH decreased to 9. Write a balanced chemical equation for the reaction and explain the decrease in pH.
ii. In another experiment solid NaHCO₃ was added to an aqueous solution of HCl. After stirring the pH increased to 5. Write a balanced equation for the reaction and explain this result.
c. Describe how the two reactions of NaHCO₃ in (b) illustrate the Brønsted–Lowry theory of acids and bases.
Medium
Mark as Complete
Mark Scheme
Question 6
Which solution is basic at 25 °C?
Kw = 1.0 × 10−14
A. [H+] = 1.0 × 10-3 mol dm-3
B. [OH-] = 1.0 × 10-13 mol dm-3
C. Solution of pH = 4.00
D. [H3O+] = 1.0 × 10-13 mol dm-3
Medium
Mark as Complete
Mark Scheme
Question 7
The strengths of organic acids can be compared using Ka and pKa values. Which acid is the strongest?
A. Acid A – pKa = 6.6
B. Acid B – pKa = 2.5
C. Acid C – Ka = 1 × 10−5
D. Acid D – Ka = 1 × 10−3
Medium
Mark as Complete
Mark Scheme
Question 8
What is the pH of 0.001 mol dm-3 NaOH (aq)?
A. 1
B. 3
C. 11
D. 13
Medium
Mark as Complete
Mark Scheme
Question 9
A 20.00 cm3 solution of the weak monoprotic acid (HA), was titrated against a solution of 0.50 mol dm-3 of sodium hydroxide in which a few drops of indicator had been added. The pH readings were not recorded until 10.00 cm3 of sodium hydroxide had been added.
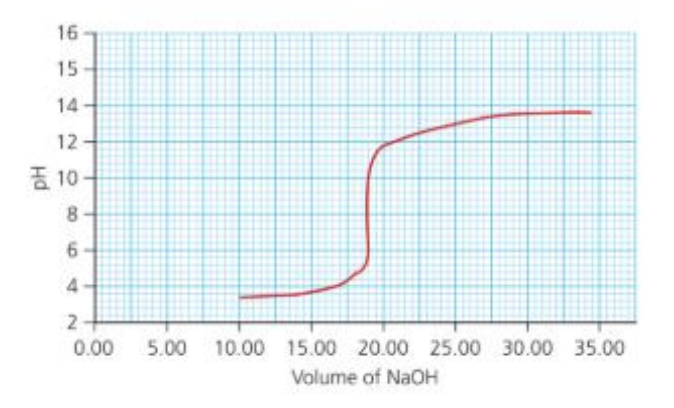
a. State the volume of sodium hydroxide needed to exactly neutralize the weak acid and hence calculate the amount of sodium hydroxide, in moles, required for neutralization.
b. Write an expression for the dissociation constant, Kₐ, of the weak acid.
c. Calculate a value for the dissociation constant, Kₐ, of the weak acid if the pH of the solution titrated is 2.10.
d. Given the following information about three indicators, state and explain which indicator is the most suitable for determining the end-point of this reaction.
| Indicator | pH range of colour change |
| Methyl red | 4.4–6.2 |
| Cresol red | 7.2–8.8 |
| Alizarin yellow | 10.1–12.0 |
Hard
Mark as Complete
Mark Scheme
Question 10
When carbon dioxide reacts with water, it forms carbonic acid, H₂CO₃, which ionizes into hydrogencarbonate ions, HCO₃⁻, and hydrogen ions, H⁺, into the sea, decreasing its pH and causing acidification.
A solution of 0.100 mol dm⁻³ H₂CO₃ has a pH of 3.68.
Due to the increasing levels of atmospheric CO₂, the pH of seawater has decreased over 150 years from 8.25 to 8.14. HCO₃⁻ and CO₃²⁻ are the essential components of the carbonate buffer system which regulates the pH of seawater.
CO₂(aq) + CO₃²⁻(aq) + H₂O(l) ⇌ 2HCO₃⁻(aq)
The natural pH of the ocean is determined by the deposition of calcium carbonate in coral reefs against the entry of calcium and carbonate ions into the ocean from weathering of limestone rocks and other minerals on land.
Ca²⁺(aq) + CO₃²⁻(aq) ⇌ CaCO₃(s)
a. Explain, with the aid of appropriate calculations, whether carbonic acid, H₂CO₃, is a strong or weak acid. Assume carbonic acid to be monoprotic in your calculations.
b. Calculate the percentage increase in the concentration of H⁺ ions in the last 150 years.
c. Suggest another environmental problem that can contribute to ocean acidification
Hard
Mark as Complete
Mark Scheme
Question 11
a. i. Define a Brønsted–Lowry acid.
ii. Deduce the two acids and their conjugate bases in the following reaction:
H₂O (l) + NH₃ (aq) ⇌ OH⁻ (aq) + NH₄⁺ (aq)
iii. Explain why the following reaction can also be described as an acid–base reaction:
F⁻ (g) + BF₃ (g) ⇌ BF₄⁻ (s)
b. Ethanoic acid, CH₃COOH, is a weak acid.
i. Define the term weak acid and state the equation for the reaction of ethanoic acid with water.
ii. Vinegar, which contains ethanoic acid, can be used to clean deposits of calcium carbonate from the elements of electric kettles. State the equation for the reaction of ethanoic acid with calcium carbonate.
Hard
Mark as Complete
Mark Scheme
Question 12
Which titration curve would occur when a weak acid is added to a strong base?\
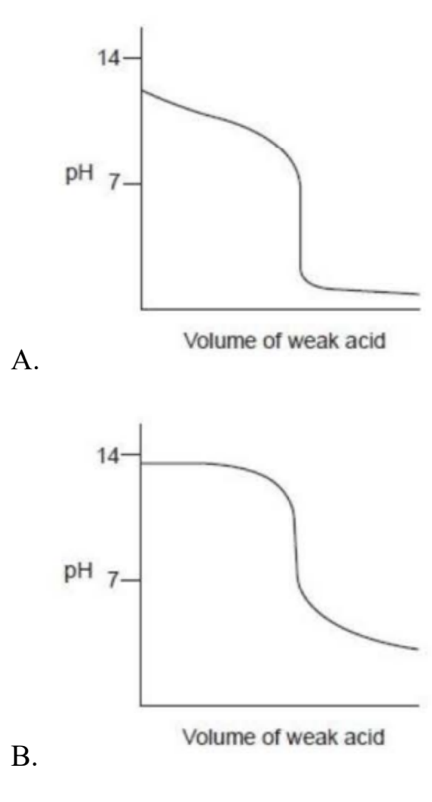
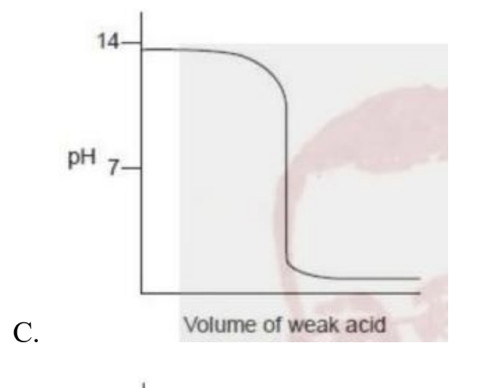
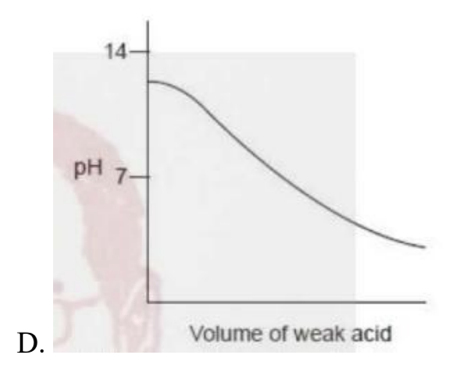
Medium
Mark as Complete
Mark Scheme
Question 13
What is the buffer region in the acid – base titration curves below?
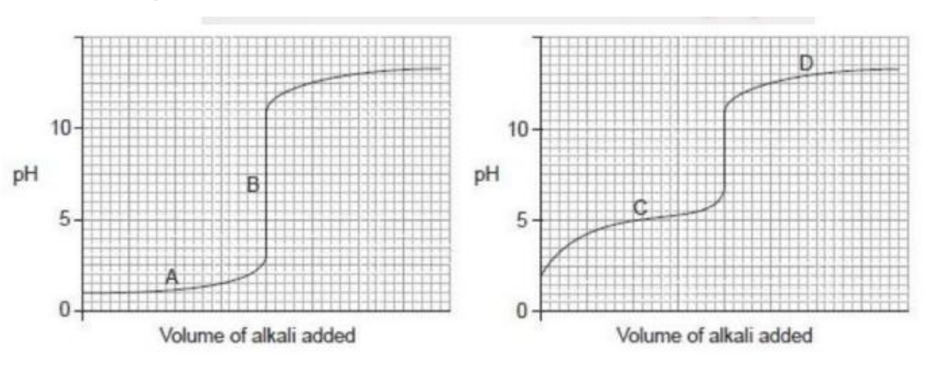
Medium
Mark as Complete
Mark Scheme
Question 14
Determine the pH of a buffer solution, correct to two decimal places, showing your working, consisting of 10.0 g of CH3COOH and 10.0 g of CH3COONa in 0.250 dm³ of solution. Ka forCH3COOH = 1.8 × 10⁻⁵ at 298 K.
Medium
Mark as Complete
Mark Scheme
Question 15
Hypochlorous acid, HOCl (aq), is an example of a weak acid.
a. State the expression for the ionic product constant of water, Kw.
b. A household bleach contains sodium hypochlorite, NaOCl (aq), at a concentration of 0.705 mol dm⁻3. The hypochlorite ion, OCl⁻ (aq), is a weak base.
OCl⁻ (aq) + H₂O (l) ⇌ HOCl (aq) + OH⁻ (aq)
i. The pKₐ value of HOCl (aq) is 7.52. Determine the Kb value of OCl⁻ (aq) assuming a temperature of 298 K.
ii. Determine the concentration of OH⁻ (aq), in mol dm⁻3, at equilibrium and state one assumption made in arriving at your answer other than a temperature of 298 K.
iii. Calculate the pH of the bleach.
Hard
Mark as Complete
Mark Scheme
Question 16
The graph below shows a computer simulation of a titration of 25.0 cm3 of 0.100 mol dm⁻3 hydrochloric acid with 0.100 mol dm⁻3 sodium hydroxide and the pH range of phenol red indicator.
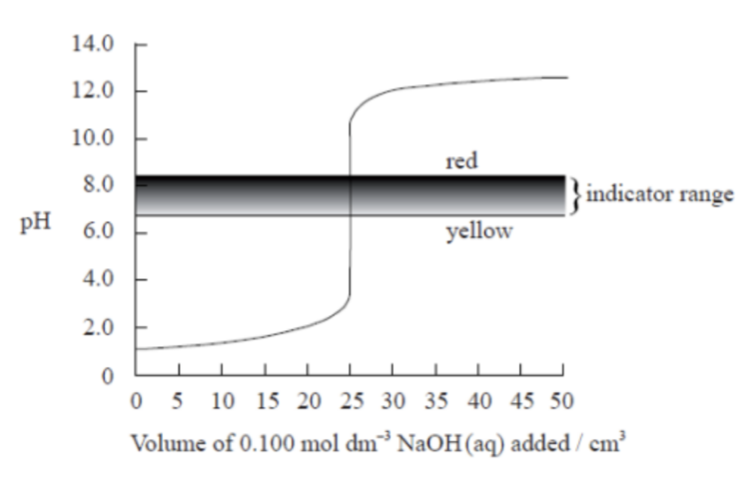
Sketch the graph that would be obtained for the titration of 25.0 cm3 of 0.100 mol dm⁻3 propanoic acid with 0.100 mol dm⁻3 potassium hydroxide using bromophenol blue as an indicator. (The pH range of bromophenol blue can be found in Table 16 of the Data Booklet).
Hard
Mark as Complete
Mark Scheme
Question 17
The pKa of ethanoic acid is 4.8 at 298 K. Which combination will produce a buffer solution with a pH of 4.8 at 298 K?
A. 20.0 cm3 of 1.0 mol dm⁻3 CH₃COOH and 10.0 cm3 of 1.0 mol dm⁻3 NaOH. B. 20.0cm3 of 1.0 mol dm⁻3 CH₃COOH and 20.0 cm3 of 1.0 mol dm⁻3 NaOH. C. 10.0 cm3 of 1.0 mol dm⁻3 CH₃COOH and 20.0 cm3 of 1.0 mol dm⁻3 NaOH. D. 14.8 cm3 of 1.0 mol dm⁻3 CH₃COOH and 10.0cm3 of 1.0 mol dm⁻3 NaOH.
Medium
Mark as Complete
Mark Scheme
Question 18
Which indicator is appropriate for the acid–base titration shown below?
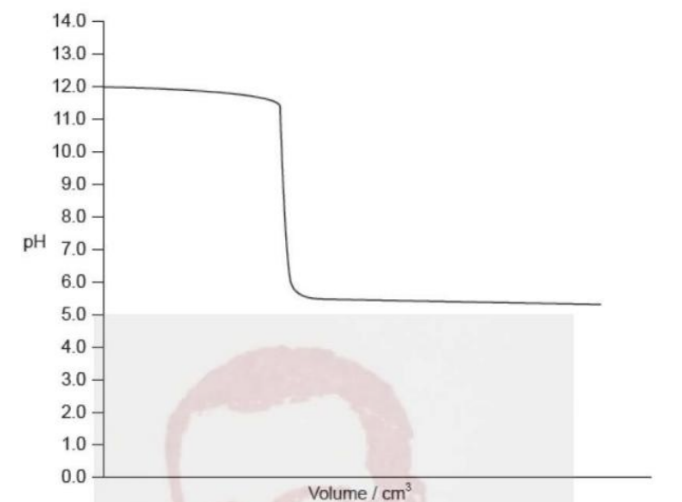
A. Thymol blue (pKₐ = 1.5)
B. Methyl orange (pKₐ = 3.7)
C. Bromophenol blue (pKₐ = 4.2)
D. Phenolphthalein (pKₐ = 9.6)
Medium
Mark as Complete
Mark Scheme
Question 19
What is a possible value of pH at the equivalence point in the titration of a strong acid with a weak base?
A. 11
B. 8
C. 7
D. 5
Easy
Mark as Complete
Mark Scheme
Question 20
The indicator bromophenol blue, HIn (aq), has a form that is yellow and an In⁻ (aq) form that is blue.
a. Write an equation to show how bromophenol blue acts as an indicator.
b. State and explain the colour of bromophenol blue:
i. On the addition of a strong acid.
ii. At the equivalence point of a titration.
Medium
Mark as Complete
Mark Scheme
Question 21
Which 1.0 mol dm⁻3 solution has the highest pH?
A. Ammonium chloride.
B. Sulfuric acid.
C. Sodium chloride.
D. Ammonia.
Medium
Mark as Complete
Mark Scheme
Question 22
10.00 cm3 of 0.01 mol dm-3 nitric acid (HNO₃) is diluted with 90.00 cm³ of water. What is the pH of the resulting solution?
A. 4
B. 3
C. 2
D. 1
Medium
Mark as Complete
Mark Scheme
Question 23
When the following 1.00 mol dm⁻3 aqueous solutions are listed in increasing order of pH (lowest first), what is the correct order?
A. HNO₃ < HCOOH < NH₃ < Ba(OH)₂.
B. NH₃ < Ba(OH)₂ < HCOOH < HNO₃.
C. Ba(OH)₂ < HCOOH < NH₃ < HNO₃.
D. HNO₃ < HCOOH < Ba(OH)₂ < NH₃.
Medium
Mark as Complete
Mark Scheme
Question 24
The amino acid alanine has the molecular structure NH₂CH(CH₃)COOH. Which of the following species represents its conjugate acid?
A. ⁺NH₃CH(CH₃)COOH
B. ⁺NH₃CH(CH₃)COOH₂⁺
C. ⁺NH₃CH(CH₃)COO⁻
D. NH₂CH(CH₃)COO⁻
Easy
Mark as Complete
Mark Scheme
Question 25
Which is a conjugate acid–base pair according to the Brønsted–Lowry theory?
CH₂ClCOOH (aq) + H₂O (l) ⇌ CH₂ClCOO⁻ (aq) + H₃O⁺ (aq)
A. H₂O / H₃O⁺
B. H₂O / CH₂ClCOO⁻
C. CH₂ClCOO⁻ / H₃O⁺
D. CH₂ClCOOH / H₂O
Easy
Mark as Complete
Mark Scheme
Question 26
A sample of benzenecarboxylic acid solution, C₆H₅COOH (aq), is diluted at constant temperature. Which diagram shows how the pH of the acid changes as it is diluted? [V is the volume of water added.]


Medium
Mark as Complete
Mark Scheme
Question 27
Under suitable conditions, NH₄I and NaNH₂ react as follows:
NH₄I + KNH₂ → KI + 2NH₃
Which term best describes the above reaction?
A. Reduction–oxidation reaction.
B. Displacement reaction.
C. Brønsted–lowry acid–base reaction.
D. Substitution reaction.
Easy
Mark as Complete
Mark Scheme
Question 28
a. Outline the Brønsted–Lowry theory of acids and bases.
b. Write a chemical equation, including state symbols, to show why rain water is slightly acidic.
c. The carbonate ion is a conjugate base of the hydrogencarbonate ion, HCO₃⁻. Define the term conjugate base.
d. Using appropriate chemical equations, show that the hydrogencarbonate ion is amphiprotic and can act as a proton donor and a proton acceptor.
Medium
Mark as Complete
Mark Scheme
Question 29
The equation for the reaction that occurs when ammonia gas dissolves in water is shown below:
NH₃(g) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
a. State how the equation indicates that ammonia is a base.
b. State how the equation indicates that ammonia is a weak base.
c. Identify which pH value is approximately correct for ammonia solution.
pH: 1 3 7 11 13
Medium
Mark as Complete
Mark Scheme
Question 30
Sodium hydrogencarbonate dissolves in water forming an alkaline solution according to the following ionic equilibrium:
HCO₃⁻(aq) + H₂O(l) ⇌ H₂CO₃(aq) + OH⁻(aq)
a. Why is the solution alkaline?
b. Using the Brønsted–Lowry theory, state, with a brief explanation, whether the HCO₃⁻ ion is behaving as an acid or as a base.
c. Identify the conjugate base of carbonic acid, H₂CO₃.
Medium
Mark as Complete
Mark Scheme
Question 31
Which of the following mixtures is not an acid / conjugate base pair?
A. H₂O / OH⁻.
B. H₂PO₄⁻ / HPO₄²⁻.
C. KH / K.
D. NH₃ / NH₂⁻.
Easy
Mark as Complete
Mark Scheme
Question 32
Hydrochloric acid is a strong acid, whereas ethanoic acid is a weak acid. What is the difference between a strong acid and a weak acid?
Medium
Mark as Complete
Mark Scheme
Question 33
Which of the following represents the reaction between zinc powder and a dilute aqueous solution of sulfuric acid?
A. Zn + 2H₂SO₄ → 2ZnS + 2H₂O + 3O₂.
B. 4Zn + H₂SO₄ → 4ZnO + H₂S.
C. Zn + H₂SO₄ → ZnSO₄ + H₂.
D. Zn + H₂SO₄ → ZnH₂ + SO₂ + O₂.
Easy
Mark as Complete
Mark Scheme
Question 34
Which ions produced by the ionization of phosphoric(V) acid, H₃PO₄, are amphiprotic?
A. HPO₄2⁻ and PO₄3⁻.
B. H₂PO₄⁻ and HPO₄2⁻.
C. HPO₄2⁻ only.
D. H₂PO₄⁻ and PO₄3⁻.
Easy
Mark as Complete
Mark Scheme
Question 35
Which is a 0.001 mol dm⁻3 solution of a weak acid?
| Conductivity | pH | |
| A. | poor | 5 |
| B. | good | 7 |
| C.. | poor | 10 |
| D. | good | 3 |
Easy
Mark as Complete
Mark Scheme
Question 36
Which gas in the atmosphere causes the pH of unpolluted rain to be approximately 6?
A. Carbon dioxide.
B. Sulfur dioxide.
C. Oxygen.
D. Nitrogen.
Easy
Mark as Complete
Mark Scheme
Question 37
Which row correctly describes 1.0 mol dm⁻3 NaOH (aq)?
| pH | Colour in universal indicator solution | Electrical conductivity | |
| A. | 14 | Purple | Good |
| B. | 10 | Green | Poor |
| C. | 14 | Red | Good |
| D. | 10 | Blue | Poor |
Medium
Mark as Complete
Mark Scheme
Question 38
a. Define the terms acid and base according to the Brønsted–Lowry theory and state one example of a weak acid and one example of a strong base.
b. Describe two different methods, one chemical and one physical, other than measuring the pH, that could be used to distinguish between ethanoic acid and hydrochloric acid solutions of the same concentration.
c. Black coffee has a pH of 5 and toothpaste has a pH of 8. Identify which is more acidic and deduce how many times the [H⁺] is greater in the more acidic product.
Medium
Mark as Complete
Mark Scheme
Question 39
Samples of sodium oxide and sulfur trioxide are added to separate beakers of water. Deduce the equation for each reaction and identify each oxide as acidic, basic or neutral.
Medium
Mark as Complete
Mark Scheme
Question 40
a. The equations of two acid–base reactions are given below.
Reaction A: NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
The reaction mixture in A consists mainly of reactants because the equilibrium lies to the left.
Reaction B: NH₂⁻(aq) + H₂O(l) ⇌ NH₃(aq) + OH⁻(aq)
The reaction mixture in B consists mainly of products because the equilibrium lies to the right.
i. For each of the reactions A and B, deduce whether water is acting as an acid or a base and explain your answer.
ii. In reaction B, identify the stronger base, NH₂⁻ or OH⁻, and explain your answer.
iii. In reactions A and B, identify the stronger acid, NH₄⁺ or NH₃ (underlined), and explain your answer.
b. Describe two different experimental methods to distinguish between aqueous solutions of a strong base and a weak base.
c. Two acidic solutions, X and Y, of equal concentrations have pH values of 2 and 6 respectively.
i. Calculate the hydrogen ion concentrations in the two solutions and identify the stronger acid.
ii. Determine the ratio of the hydrogen ion concentrations in the two solutions X and Y.
Hard
Mark as Complete
Mark Scheme
Question 1
What is the order of increasing acidity?
| Acid | pKₐ | Acid | Kₐ |
| HClO | 7.4 | HF | 5.6 × 10⁻⁴ |
| HIO₃ | 0.8 | CH₃CH₂COOH | 1.3 × 10⁻⁵ |
A. HClO < CH₃CH₂COOH < HF < HIO₃
B. HClO < HF < CH₃CH₂COOH < HIO₃
C. HIO₃ < HF < CH₃CH₂COOH < HClO
D. HIO₃ < CH₃CH₂COOH< HF < HClO
Answer: A. HClO <CH₃CH₂COOH < HF < HIO₃
• Lower pKa → stronger acid
• Higher Ka → stronger acid
| Acid | Relative strength |
| HIO₃ | pKa 0.8 → very strong |
| HF | Ka 5.6 × 10⁻⁴ → stronger than propanoic acid |
| CH₃CH₂COOH | Ka 1.3 × 10⁻⁵ → weaker acid |
| HClO | pKa 7.4 → weakest |
Arrange in increasing acidity (weakest → Strongest)
HClO <CH₃CH₂COOH < HF < HIO₃
Question 2
a. Explain why a 1.0 mol dm⁻3 solution of sodium hydroxide has a pH of 14 whereas 1.0 mol dm⁻3 ammonia solution has a pH of about 12. Use equations in your answer.
b. 20.0 cm3 of a known concentration of sodium hydroxide is titrated with a solution of nitric acid. The graph for this titration is given below.

i. State an equation for the reaction between sodium hydroxide and nitric acid.
ii. Calculate the concentration of the sodium hydroxide solution before the titration.
iii. From the graph determine the volume of nitric acid required to neutralize the sodium hydroxide and calculate the concentration of the nitric acid.
iv. Predict the volume of ethanoic acid of the same concentration as the nitric acid in (b)(iii), required to neutralize 20.0 cm3 of this sodium hydroxide solution.
a.
• NaOH (strong base): fully dissociates in water
NaOH(aq) → Na+(aq) + OH−(aq)
So [OH−] ≈ 1.0 mol dm⁻3 ⇒ pOH = 0 ⇒ pH = 14.
• NH3 (weak base): only partly reacts with water
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH−(aq)
Giving a much smaller [OH−] than 1.0 M, so pH ≈ 12.
b. i.
NaOH(aq) + HNO3(aq) → NaNO3(aq) + H2O(l)
ii. From the initial pH ≈ 13:
[OH−] = 10−1 = 0.10 mol dm−3 ⇒ c(NaOH) = 0.10 mol dm−3
iii. Equivalence volume (middle of the vertical drop) from the graph ≈ 18.0 cm3 of HNO₃.
Moles NaOH initially:
`frac{0.10*20.0 cm^3}{1000}`= 2.00 × 10−3 mol
At equivalence:
`""n_(HNO_3)` = 2.00 × 10−3 mol
`""c_(HNO_3) = frac{2.00*10^-3}{18.0*10^-3}` ≈ 0.11 mol dm−3
iv. Ethanoic acid is monoprotic too. If it has the same concentration as the nitric acid in (iii), the same moles are required — hence, the same volume: ≈ 18.0 cm³.
Question 3
The graph below shows the change in pH when aqueous sodium hydroxide is added to 20 cm³ of aqueous hydrochloric acid.

By reference to the graph:
a. State the [H⁺] before any alkali is added.
b. State by how much the [H⁺] changes after the addition of 20 cm³ of aqueous sodium hydroxide.
c. Determine the volume of the same sodium hydroxide solution needed to neutralize 20 cm³ of aqueous ethanoic acid of the same concentration as the hydrochloric acid.
a. Initial pH ≈ 1, so
[H+]0 = 10−1 ≈ 0.10 mol dm−3.
b. At V = 20 cm3 the pH ≈ 2, so [H+ ≈ 10−2 mol dm−3. Hence [H+] has decreased by a factor of ~10 (from ~0.10 to ~0.01 mol dm⁻3).
c. The equivalence point from the graph is at about 23 cm3 of NaOH. Ethanoic acid is monoprotic and at the same concentration as the HCl, so it will require the same volume of this NaOH: ≈ 23 cm3.
Question 4
Carbonic acid can be used to treat wasp (an insect) stings.
a. Suggest what this indicates about the nature of wasp stings.
b. Name the type of reaction that occurs.
c. Explain why hydrochloric acid is not used to treat wasp stings.
a. Since carbonic acid (H₂CO₃) is an acid, it is used to neutralize an alkaline substance. Therefore, wasp stings are alkaline in nature.
b. The reaction that occurs between an acid and an alkali is a neutralization reaction.
Acid + Base → Salt + Water
c. Hydrochloric acid is too strong – it would cause skin burns and tissue damage. Carbonic acid is weak and safe, so it can neutralize the alkali gently without harming the skin.
Question 5
Five unlabelled bottles are known to contain the following 0.10 mol dm⁻³ aqueous solutions:
CH₃COOH, NaCl, NaOH, HCl, NH₃
a. Describe and explain how the pH values of these five solutions could be used to identify them.
b. Experiments were conducted to illustrate some properties of sodium hydrogencarbonate, NaHCO₃.
i. In one experiment some solid NaHCO₃ was added to aqueous NaOH. After stirring the pH decreased to 9. Write a balanced chemical equation for the reaction and explain the decrease in pH.
ii. In another experiment solid NaHCO₃ was added to an aqueous solution of HCl. After stirring the pH increased to 5. Write a balanced equation for the reaction and explain this result.
c. Describe how the two reactions of NaHCO₃ in (b) illustrate the Brønsted–Lowry theory of acids and bases.
a. Using pH to identify the 0.10 mol dm⁻3 solutions
Measure pH with a pH meter / universal indicator and match to strengths:
| Bottle | Nature | Expected pH (≈) | Reason |
| HCl | Strong acid | 1 | Complete dissociation → high [H⁺] |
| CH₃COOH | Weak acid | ≈3 | Partial dissociation (Kₐ ≈ 1.8×10⁻⁵) |
| NaCl | Neutral salt | 7 | From strong acid/strong base → no hydrolysis |
| NH₃ | Weak base | ≈11 | Partial reaction with water to give NH₄⁺/OH⁻ |
| NaOH | Strong base | 13–14 | Complete dissociation → high [OH⁻] |
Lowest pH = HCl; next = CH₃COOH; 7 = NaCl; ~11 = NH₃; highest = NaOH. b. i. Add solid NaHCO₃ to NaOH (aq)
Balanced equation: NaHCO₃ + NaOH → Na₂CO₃ + H₂O
Explanation: OH⁻ is consumed and replaced by the weaker basic carbonate; the solution becomes less alkaline, so pH falls (to about 9).
ii. Add solid NaHCO₃ to HCl (aq)
Balanced equation: NaHCO₃ + HCl → NaCl + CO₂ + H₂O
Explanation: H⁺ is neutralized (consumed) to form CO₂ and water, so the solution becomes less acidic and the pH rises (to about 5).
c. Brønsted–Lowry interpretation
• In (b)(i): HCO₃⁻ donates a proton to OH⁻ (forming H₂O) → acts as an acid. Net ionic: HCO₃⁻ + OH⁻ → CO₃²⁻ + H₂O
• In (b)(ii): HCO₃⁻ accepts a proton from H⁺ (forming H₂CO₃ → CO₂ + H₂O) → acts as a base.
Thus HCO₃⁻ is amphiprotic, behaving as a Brønsted–Lowry acid in (i) and as a Brønsted–Lowry base in (ii).
Question 6
Which solution is basic at 25 °C?
Kw = 1.0 × 10−14
A. [H+] = 1.0 × 10-3 mol dm-3
B. [OH-] = 1.0 × 10-13 mol dm-3
C. Solution of pH = 4.00
D. [H3O+] = 1.0 × 10-13 mol dm-3
Answer: D. [H3O+] = 1.0 × 10-13 mol dm-3
A. Incorrect:
[H+] = 1.0 × 10−3
[OH-] = `frac{K_w}{[H^+]}=frac{1.0*10^(-14)}{1.0*10^(-3)}=1.0*10^(-11)`
→ [OH-] < [H+]→ acidic
B. Incorrect:
[OH-] = 1.0 × 10−13
[H+] = `frac{K_w}{[OH^-]}=frac{1.0*10^(-14)}{1.0*10^(-13)}`= 0.1
→ [H+] > [OH-]→ acidic
C. Incorrect:
pH = 4 → [H+] = 10−4
[OH-] = `frac{1.0*10^(-14)}{10^(-4)}`= 10-10
→ [OH-] < [H+]→ acidic
D. Correct:
[H3O+] = 1.0 × 10−13
[OH-] = `frac{1.0*10^(-14)}{1.0*10^(-13)}`= 0.1
→ [OH-] > [H+]→ basic
Question 7
The strengths of organic acids can be compared using Ka and pKa values. Which acid is the strongest?
A. Acid A – pKa = 6.6
B. Acid B – pKa = 2.5
C. Acid C – Ka = 1 × 10−5
D. Acid D – Ka = 1 × 10−3
Answer: B. Acid B – pKa = 2.5.
Acid Strength Relationships
• Lower pKa → stronger acid
• Higher Ka → stronger acid
Acid Strength Comparison Table
| Acid | pKa | Ka | Strength |
| A. Incorrect | 6.6 | — | Weak |
| B. Correct | 2.5 | — | Stronger |
| C. Incorrect | — | 1 × 10⁻⁵ | Weak |
| D. Incorrect | — | 1 × 10⁻³ | Stronger |
• Acid B (pKa = 2.5) → Ka ≈ 10-2.5 ≈ 3.2 × 10-3
• Acid D (Ka = 1 × 10-3)
These two are similar in strength, but acid B has the slightly higher Ka (≈ 3 × 10-3) → stronger acid.
Question 8
What is the pH of 0.001 mol dm-3 NaOH (aq)?
A. 1
B. 3
C. 11
D. 13
Answer: C. 11
[NaOH] = 0.001 mol dm⁻3 = 1.0 × 10⁻3 mol dm⁻3
NaOH is a strong base, so it dissociates completely:
[OH⁻] = 1.0 × 10⁻3
Step 1: Find pOH
pOH = −log₁₀[OH⁻] = −log₁₀(1.0 × 10⁻3) = 3
Step 2: Find pH
pH = 14 − pOH = 14 − 3 = 11
Question 9
A 20.00 cm3 solution of the weak monoprotic acid (HA), was titrated against a solution of 0.50 mol dm-3 of sodium hydroxide in which a few drops of indicator had been added. The pH readings were not recorded until 10.00 cm3 of sodium hydroxide had been added.

a. State the volume of sodium hydroxide needed to exactly neutralize the weak acid and hence calculate the amount of sodium hydroxide, in moles, required for neutralization.
b. Write an expression for the dissociation constant, Kₐ, of the weak acid.
c. Calculate a value for the dissociation constant, Kₐ, of the weak acid if the pH of the solution titrated is 2.10.
d. Given the following information about three indicators, state and explain which indicator is the most suitable for determining the end-point of this reaction.
| Indicator | pH range of colour change |
| Methyl red | 4.4–6.2 |
| Cresol red | 7.2–8.8 |
| Alizarin yellow | 10.1–12.0 |
a. From the graph the midpoint of the vertical rise (equivalence) is at ≈ 20.0 cm3 of NaOH.
b. For HA ⇌ H⁺ + A⁻,
Ka = `frac{[H^+][A^-]}{[HA]`
c. Initial acid concentration: at equivalence moles HA = 1.00 ×10-2 in 20.0 cm3 →
CHA = `frac{0.0100}{0.0200}`= 0.50 mol dm-3
Given pH = 2.10 ⇒ [H⁺] = 10−2.10 = 7.94 × 10-3 mol dm-3.
Assuming weak dissociation,
[A⁻] ≈ [H⁺] = 7.94 × 10-3 and [HA] ≈ 0.50 − 0.00794 = 0.492.
Ka = `frac{(7.94*10^(-3))^2}{0.492}`≈ 1.3 × 10-4
d. Best indicator: cresol red (pH 7.2–8.8), because its transition range lies within the steep vertical region around the equivalence point of a weak acid–strong base titration. (Methyl red changes too early; alizarin yellow too late.)
Question 10
When carbon dioxide reacts with water, it forms carbonic acid, H₂CO₃, which ionizes into hydrogencarbonate ions, HCO₃⁻, and hydrogen ions, H⁺, into the sea, decreasing its pH and causing acidification.
A solution of 0.100 mol dm⁻³ H₂CO₃ has a pH of 3.68.
Due to the increasing levels of atmospheric CO₂, the pH of seawater has decreased over 150 years from 8.25 to 8.14. HCO₃⁻ and CO₃²⁻ are the essential components of the carbonate buffer system which regulates the pH of seawater.
CO₂(aq) + CO₃²⁻(aq) + H₂O(l) ⇌ 2HCO₃⁻(aq)
The natural pH of the ocean is determined by the deposition of calcium carbonate in coral reefs against the entry of calcium and carbonate ions into the ocean from weathering of limestone rocks and other minerals on land.
Ca²⁺(aq) + CO₃²⁻(aq) ⇌ CaCO₃(s)
a. Explain, with the aid of appropriate calculations, whether carbonic acid, H₂CO₃, is a strong or weak acid. Assume carbonic acid to be monoprotic in your calculations.
b. Calculate the percentage increase in the concentration of H⁺ ions in the last 150 years.
c. Suggest another environmental problem that can contribute to ocean acidification
a. For 0.100 mol dm⁻³ H₂CO₃, pH = 3.68 → [H⁺] = 10−3.68 = 2.09 × 10−4 mol dm−3. Degree of ionization (treating as monoprotic):
`alpha = frac{[H^+]}{C}=frac{2.09*10^(-4)}{0.100}`≈ 2.1 × 10−3(0.21%)
Very small → weak acid.
`""K_alpha≈frac{x^2}{C-x}≈frac{(2.09*10^(-4))^2}{0.100}`= 4.4 × 10−7, pKa ≈ 6.36
b. pH fell from 8.25 to 8.14.
[H⁺]8.25 = 10−8.25 = 5.62 × 10−9
[H⁺]8.14 = 10−8.14 = 7.24 × 10−9
Percentage increase:
`frac{7.24-5.62}{5.62}*100%`≈ 29%
c. Another contributor: acid rain (from SO₂/NOₓ emissions) entering the oceans/coastal waters, or nutrient runoff causing eutrophication and excess CO₂ from decomposition.
Question 11
a. i. Define a Brønsted–Lowry acid.
ii. Deduce the two acids and their conjugate bases in the following reaction:
H₂O (l) + NH₃ (aq) ⇌ OH⁻ (aq) + NH₄⁺ (aq)
iii. Explain why the following reaction can also be described as an acid–base reaction:
F⁻ (g) + BF₃ (g) ⇌ BF₄⁻ (s)
b. Ethanoic acid, CH₃COOH, is a weak acid.
i. Define the term weak acid and state the equation for the reaction of ethanoic acid with water.
ii. Vinegar, which contains ethanoic acid, can be used to clean deposits of calcium carbonate from the elements of electric kettles. State the equation for the reaction of ethanoic acid with calcium carbonate.
a. i. A Brønsted–Lowry acid is a proton (H⁺) donor.
ii.
H₂O (l) + NH₃ (aq) ⇌ OH⁻ (aq) + NH₄⁺ (aq)
• Acids: H₂O and NH₄⁺
• Their conjugate bases: OH⁻ (from H₂O) and NH₃ (from NH₄⁺)
iii. F⁻ + BF₃ ⇌ BF₄⁻ is a Lewis acid–base reaction:
F⁻ donates an electron pair (Lewis base) to BF₃, which accepts it (Lewis acid), forming BF₄⁻.
b. i. A weak acid only partially ionizes in water.
CH₃COOH(aq) + H₂O(l) ⇌ H₃O+(aq) + CH₃COO⁻(aq)
ii. Reaction of ethanoic acid with calcium carbonate (descaling):
2CH₃COOH(aq) + CaCO₃(s) → (CH₃COO)₂Ca(aq) + CO₂(g) + H₂O(l)
Question 12
Which titration curve would occur when a weak acid is added to a strong base?\



Answer: B.
A strong base, so the pH is high (~14). As a weak acid is added, pH drops gradually (buffering), then there’s a relatively sharp fall with the equivalence point above pH 7 (solution contains the conjugate base of the weak acid). With excess weak acid, the curve levels off around pH ~3–4—not near 0. Curve B matches this behavior.
Question 13
What is the buffer region in the acid – base titration curves below?

Answer: C.
The buffer region in an acid-base titration curve is the area where the pH of the solution changes very gradually as the titrant (acid or base) is added. This happens because the solution contains a mixture of a weak acid and its conjugate base (or a weak base and its conjugate acid), which can resist large changes in pH.
In the provided graphs: The first curve shows the titration of a strong acid with a strong base, which does not have a buffer region. The pH changes very rapidly once the titration begins and around the equivalence point. The second curve represents the titration of a weak acid with a strong base. The region labeled C is the buffer region, where the pH rises slowly and the solution is most effective at resisting pH changes.
Question 14
Determine the pH of a buffer solution, correct to two decimal places, showing your working, consisting of 10.0 g of CH3COOH and 10.0 g of CH3COONa in 0.250 dm³ of solution. Ka forCH3COOH = 1.8 × 10⁻⁵ at 298 K.
Given
Concentrations:
[HA] = `frac{0.1665}{0.250}`= 0.666 M
[A-] = `frac{0.1219}{0.250}`= 0.488 M
Use Henderson–Hasselbalch:
pH = pKa + `logfrac{[A^-]}{[HA]}`= 4.7447 + `logfrac{0.488}{0.666}`= 4.7447 + log (0.733) ≈ 4.61
Question 15
Hypochlorous acid, HOCl (aq), is an example of a weak acid.
a. State the expression for the ionic product constant of water, Kw.
b. A household bleach contains sodium hypochlorite, NaOCl (aq), at a concentration of 0.705 mol dm⁻3. The hypochlorite ion, OCl⁻ (aq), is a weak base.
OCl⁻ (aq) + H₂O (l) ⇌ HOCl (aq) + OH⁻ (aq)
i. The pKₐ value of HOCl (aq) is 7.52. Determine the Kb value of OCl⁻ (aq) assuming a temperature of 298 K.
ii. Determine the concentration of OH⁻ (aq), in mol dm⁻3, at equilibrium and state one assumption made in arriving at your answer other than a temperature of 298 K.
iii. Calculate the pH of the bleach.
a. Kw = [H3O⁺][OH⁻] (or [H⁺][OH⁻])
b. For OCl⁻ + H₂O ⇌ HOCl + OH⁻
i. pKa(HOCl) = 7.52 ⇒ Ka = 10-7.52 = 3.02 × 10⁻⁸
Kb (OCl⁻) = `frac{K_w}{K_a}`= `frac{1.0*10^(-14)}{3.02*10^(-8)}`= 3.31 × 10-7
ii. For initial [OCl⁻] = 0.705 mol dm⁻3, let x = [OH⁻] at equilibrium.
Kb = `frac{x^2}{0.705-x}` ≈`frac{x^2}{0.705}` ⇒ x = `sqrt(K_b*0.705)`= 4.83 × 10⁻⁴ mol dm⁻3
Assumption: the base is only slightly ionised so 0.705 − x ≈ 0.705 (and water autoionisation is neglected).
iii. pOH = −log (4.83 × 10⁻⁴) = 3.316 ⇒ pH = 14 − pOH = 10.68
Question 16
The graph below shows a computer simulation of a titration of 25.0 cm3 of 0.100 mol dm⁻3 hydrochloric acid with 0.100 mol dm⁻3 sodium hydroxide and the pH range of phenol red indicator.

Sketch the graph that would be obtained for the titration of 25.0 cm3 of 0.100 mol dm⁻3 propanoic acid with 0.100 mol dm⁻3 potassium hydroxide using bromophenol blue as an indicator. (The pH range of bromophenol blue can be found in Table 16 of the Data Booklet).

Weak acid (propanoic acid, CH₃CH₂COOH) titrated with strong base (KOH), using bromophenol blue as indicator.
Step 1: Understand acid–base strength
• Propanoic acid is weak → partially dissociates.
• KOH is strong → fully dissociates.
Therefore, the equivalence point will be basic (pH > 7) because the conjugate base (propanoate ion) hydrolyzes in water.
Step 2: Key points on the graph
| Stage | Description | Approx. pH |
| Start | Only weak acid present | pH ≈ 3 |
| Buffer region | Gradual rise as acid partially neutralized | pH 3–6 |
| Half-neutralization point | [HA] = [A⁻] → pH = pKa of propanoic acid (~4.9) | pH ≈ 4.9 |
| Equivalence point | All acid converted to A⁻ → basic solution | pH ≈ 8.5–9 |
| Excess base | pH rises sharply then levels off | pH ≈ 12–13 |
Step 3: Indicator range
Bromophenol blue: pH range ≈ 3.0–4.6 (changes from yellow to blue) → The indicator changes too early, before the equivalence point (which is around pH 8.5–9). So, bromophenol blue is not suitable for this titration — it would change color before the endpoint.
Step 4: Sketch description
• Start pH ≈ 3 (higher than strong acid).
• Slowly rises forming a buffer region (gentle slope).
• Sharp rise around 25 cm³, but less vertical and shifted to right, equivalence near pH 8.5.
• Then curve flattens above pH 12.
Shade the indicator range (3.0–4.6) — on the left side, far below the vertical section. Label:
• “Indicator changes too early.”
• “Equivalence ≈ pH 8.5 (basic).”
Question 17
The pKa of ethanoic acid is 4.8 at 298 K. Which combination will produce a buffer solution with a pH of 4.8 at 298 K?
A. 20.0 cm3 of 1.0 mol dm⁻3 CH₃COOH and 10.0 cm3 of 1.0 mol dm⁻3 NaOH. B. 20.0cm3 of 1.0 mol dm⁻3 CH₃COOH and 20.0 cm3 of 1.0 mol dm⁻3 NaOH. C. 10.0 cm3 of 1.0 mol dm⁻3 CH₃COOH and 20.0 cm3 of 1.0 mol dm⁻3 NaOH. D. 14.8 cm3 of 1.0 mol dm⁻3 CH₃COOH and 10.0cm3 of 1.0 mol dm⁻3 NaOH.
Answer: A. 20.0 cm3 of 1.0 mol dm-3 CH₃COOH and 10.0 cm3 of 1.0 mol dm-3 NaOH.
For a buffer with pH = pKa (4.8), we need [A−] = [HA].
A. Correct:
20 mmol CH₃COOH with 10 mmol NaOH ⇒ neutralize 10 mmol ⇒ left: 10 mmol HA and 10 mmol A⁻ → [A−] = [HA]⇒ pH = pKa = 4.8.
B. Incorrect:
20 mmol acid + 20 mmol base ⇒ complete neutralization (only A⁻).
C. Incorrect:
Base in excess ⇒ [A−] ≠ [HA].
D. Incorrect:
Base in unequal amounts ⇒ [A−] ≠ [HA].
Question 18
Which indicator is appropriate for the acid–base titration shown below?

A. Thymol blue (pKₐ = 1.5)
B. Methyl orange (pKₐ = 3.7)
C. Bromophenol blue (pKₐ = 4.2)
D. Phenolphthalein (pKₐ = 9.6)
Answer: C. Bromophenol blue (pKₐ = 4.2)
1. Type of titration
• The pH starts high (~12) → solution is basic initially (so a base is in the flask).
• The pH ends low (~3–4) → acid is added.
• The equivalence point (steep drop) occurs around pH 7–9 → below 7, suggesting this is a strong base + weak acid titration (but actually the opposite — check the curve).
Here, because the pH starts high and drops sharply below 7, this is a strong base being titrated by a strong acid, or a weak base titrated by a strong acid.
The equivalence point is around pH 5–6, so the titration is weak base + strong acid.
2. Suitable indicator
We need an indicator that changes color around pH 5–6, where the equivalence point occurs.
| Option | Indicator | pKa | Approx. range | Suitability |
| A. Incorrect | Thymol blue | 1.5 | 1.2–2.8 | Too low |
| B. Correct | Methyl orange | 3.7 | 3.1–4.4 | Slightly below equivalence, close |
| C. Incorrect | Bromophenol blue | 4.2 | 3.0–4.6 | Also close |
| D. Incorrect | Phenolphthalein | 9.6 | 8.3–10.0 | Too high |
Between methyl orange and bromophenol blue, bromophenol blue is better, since it changes closer to pH 4–5, matching the equivalence region.
Question 19
What is a possible value of pH at the equivalence point in the titration of a strong acid with a weak base?
A. 11
B. 8
C. 7
D. 5
Answer: D. 5
For a titration of a strong acid with a weak base, the solution at the equivalence point contains the conjugate acid of the weak base (which is slightly acidic). Thus, pH < 7 — typically around 5.
Question 20
The indicator bromophenol blue, HIn (aq), has a form that is yellow and an In⁻ (aq) form that is blue.
a. Write an equation to show how bromophenol blue acts as an indicator.
b. State and explain the colour of bromophenol blue:
i. On the addition of a strong acid.
ii. At the equivalence point of a titration.
a. HIn(aq) ⇌ H+(aq) + In−(aq)
Yellow ⇌ Blue
b. i. On addition of a strong acid:
Adding acid increases [H+], shifting equilibrium to the left (Le Chatelier’s principle).
→ More HIn forms → solution turns yellow.
ii. At the equivalence point of a titration:
At the equivalence point, [HIn] ≈ [In−].
→ Both forms are present in comparable amounts → the colour is green (intermediate between yellow and blue).
Question 21
Which 1.0 mol dm⁻3 solution has the highest pH?
A. Ammonium chloride.
B. Sulfuric acid.
C. Sodium chloride.
D. Ammonia.
Answer: D. Ammonia.
A. Incorrect: Ammonium chloride (NH₄Cl)
• Contains NH₄⁺, a weak acid (conjugate acid of NH₃).
• Solution is acidic (pH < 7).
B. Incorrect: Sulfuric acid (H₂SO₄)
• Strong acid.
• Very low pH (~0–1).
C. Incorrect: Sodium chloride (NaCl)
• Salt of a strong acid and strong base.
• Neutral solution, pH ≈ 7.
D. Correct: Ammonia (NH₃)
• Weak base.
• Forms NH₄⁺ and OH⁻ in water.
• Basic solution, pH ≈ 11.
Question 22
10.00 cm3 of 0.01 mol dm-3 nitric acid (HNO₃) is diluted with 90.00 cm³ of water. What is the pH of the resulting solution?
A. 4
B. 3
C. 2
D. 1
Answer: B. 3
Total volume = 10 + 90 = 100.00 cm3
Step 1: Find moles of HNO₃
n = C × V = 0.01 × `frac{10}{1000}`= 0.0001 mol
Step 2: Find new concentration after dilution
Cnew = `frac{n}{V_"total"} = frac{0.0001}{frac{100}{1000}}`= 0.0001 mol dm-3
Step 3: Determine [H⁺]
Since HNO₃ is a strong acid, it dissociates completely:
[H+] = 0.001 mol dm-3
Step 4: Calculate pH
pH = −log [H+] = −log (0.001) = 3
Question 23
When the following 1.00 mol dm⁻3 aqueous solutions are listed in increasing order of pH (lowest first), what is the correct order?
A. HNO₃ < HCOOH < NH₃ < Ba(OH)₂.
B. NH₃ < Ba(OH)₂ < HCOOH < HNO₃.
C. Ba(OH)₂ < HCOOH < NH₃ < HNO₃.
D. HNO₃ < HCOOH < Ba(OH)₂ < NH₃.
Answer: A. HNO₃ < HCOOH < NH₃ < Ba(OH)₂.
1. HNO₃ – strong acid → very low pH (~0)
2. HCOOH (formic acid) – weak acid → pH around 2–3
3. NH₃ – weak base → pH around 11
4. Ba(OH)₂ – strong base → produces 2 OH⁻ per formula unit → pH very high (~14) Increasing order of pH (lowest → highest): HNO₃ < HCOOH < NH₃ < Ba(OH)₂
Question 24
The amino acid alanine has the molecular structure NH₂CH(CH₃)COOH. Which of the following species represents its conjugate acid?
A. ⁺NH₃CH(CH₃)COOH
B. ⁺NH₃CH(CH₃)COOH₂⁺
C. ⁺NH₃CH(CH₃)COO⁻
D. NH₂CH(CH₃)COO⁻
Answer: A. ⁺NH₃CH(CH₃)COOH
NH₂CH(CH₃)COOH contains:
• –NH₂ (basic site)
• –COOH (acidic site)
Conjugate acid concept: A conjugate acid is formed when a species gains a proton (H⁺). Alanine can gain a proton on its basic –NH₂ group, forming –NH₃⁺. ⇒ NH₂CH(CH₃)COOH + H⁺ → ⁺NH₃CH(CH₃)COOH
A. Correct: ⁺NH₃CH(CH₃)COOH
Proton added to the amino group
B. Incorrect: ⁺NH₃CH(CH₃)COOH₂⁺
Too many protons – both groups protonated (diprotic form)
C. Incorrect: ⁺NH₃CH(CH₃)COO⁻
Zwitterion, not conjugate acid (same number of H⁺ as neutral form)
D. Incorrect: NH₂CH(CH₃)COO⁻
Conjugate base, not acid
Question 25
Which is a conjugate acid–base pair according to the Brønsted–Lowry theory?
CH₂ClCOOH (aq) + H₂O (l) ⇌ CH₂ClCOO⁻ (aq) + H₃O⁺ (aq)
A. H₂O / H₃O⁺
B. H₂O / CH₂ClCOO⁻
C. CH₂ClCOO⁻ / H₃O⁺
D. CH₂ClCOOH / H₂O
Answer: A. H₂O / H₃O⁺
Identify proton transfer
• CH₂ClCOOH donates a proton (H⁺) → acts as a Brønsted–Lowry acid.
• H₂O accepts a proton → acts as a Brønsted–Lowry base.
After transfer:
• CH₂ClCOOH → CH₂ClCOO⁻ (its conjugate base)
• H₂O → H₃O⁺ (its conjugate acid)
Therefore, the conjugate acid–base pair is: H₂O / H₃O⁺
Question 26
A sample of benzenecarboxylic acid solution, C₆H₅COOH (aq), is diluted at constant temperature. Which diagram shows how the pH of the acid changes as it is diluted? [V is the volume of water added.]


Answer: B.
Step 1: What happens to concentration?
As you dilute the acid:
• The acid concentration decreases.
• Therefore, the concentration of H⁺ ions also decreases.
Step 2: What happens to pH?
Since pH = −log[H⁺], as [H⁺] decreases → pH increases (solution becomes less acidic).
Step 3: Shape of the curve
• The increase in pH is gradual, not linear.
• At high dilution, the pH increase levels off because water’s autoionization limits further change.
Thus, the pH increases slowly and asymptotically, remaining below 7 (since it’s still an acidic solution).
Question 27
Under suitable conditions, NH₄I and NaNH₂ react as follows:
NH₄I + KNH₂ → KI + 2NH₃
Which term best describes the above reaction?
A. Reduction–oxidation reaction.
B. Displacement reaction.
C. Brønsted–lowry acid–base reaction.
D. Substitution reaction.
Answer: C. Brønsted–lowry acid–base reaction.
A. Incorrect: No oxidation state change → not redox.
B. Incorrect: No ion displacement → not displacement.
C. Correct:
• NH₄⁺ (from NH₄I) is the acid — it can donate a proton (H⁺).
• NH₂⁻ (from KNH₂) is the base — it can accept a proton.
NH₄⁺ + NH₂⁻ → 2NH₃
That’s a proton transfer reaction — the defining feature of a Brønsted–Lowry acid–base reaction.
D. Incorrect: No atom or group substitution → not substitution.
Question 28
a. Outline the Brønsted–Lowry theory of acids and bases.
b. Write a chemical equation, including state symbols, to show why rain water is slightly acidic.
c. The carbonate ion is a conjugate base of the hydrogencarbonate ion, HCO₃⁻. Define the term conjugate base.
d. Using appropriate chemical equations, show that the hydrogencarbonate ion is amphiprotic and can act as a proton donor and a proton acceptor.
a. Brønsted–Lowry: acids are proton (H⁺) donors, bases are proton (H⁺) acceptors.
b. Reason rainwater is slightly acidic
CO2(g) + H2O(l) ⇌ H+(aq) + HCO3−(aq)
(Equivalently:
CO2(g) + H2O(l) ⇌ H2CO3(aq)
H2CO3(aq) ⇌ H+(aq) + HCO3−(aq) )
c. Conjugate base: the species formed when an acid donates one proton (differ from the acid by −H+), and can accept the proton back.
d. Amphiprotic behavior of HCO3−:
• As an acid (proton donor):
HCO3−(aq) ⇌ H+(aq) + CO32−(aq)
(or HCO3− + OH− → CO32− + H2O)
• As a base (proton acceptor):
HCO3−(aq) + H+(aq) → H2CO3(aq)
Question 29
The equation for the reaction that occurs when ammonia gas dissolves in water is shown below:
NH₃(g) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
a. State how the equation indicates that ammonia is a base.
b. State how the equation indicates that ammonia is a weak base.
c. Identify which pH value is approximately correct for ammonia solution.
pH: 1 3 7 11 13
a. How the equation indicates that ammonia is a base
NH₃(g) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
• Ammonia accepts a proton (H⁺) from water to form NH₄⁺.
• By Brønsted–Lowry theory, a base is a proton acceptor.
→ Ammonia acts as a base because it accepts a proton (H⁺) from water.
b. How the equation indicates that ammonia is a weak base
• The equation has a reversible (⇌) arrow.
• This shows partial ionization – only some NH₃ molecules react with water.
→ The equilibrium sign shows that ammonia is only partially ionized, so it is a weak base.
c. Approximate pH of ammonia solution
• Aqueous ammonia is weakly basic, so its pH is greater than 7, but not as high as strong bases like NaOH.
• Typically, pH ≈ 11.
Question 30
Sodium hydrogencarbonate dissolves in water forming an alkaline solution according to the following ionic equilibrium:
HCO₃⁻(aq) + H₂O(l) ⇌ H₂CO₃(aq) + OH⁻(aq)
a. Why is the solution alkaline?
b. Using the Brønsted–Lowry theory, state, with a brief explanation, whether the HCO₃⁻ ion is behaving as an acid or as a base.
c. Identify the conjugate base of carbonic acid, H₂CO₃.
a. Why is the solution alkaline?
From the equation:
HCO₃⁻(aq) + H₂O(l) ⇌ H₂CO₃(aq) + OH⁻(aq)
Hydroxide ions (OH⁻) are produced. Since OH⁻ ions make the solution basic (pH > 7), the solution is alkaline.
→ Because the equilibrium produces hydroxide ions (OH⁻), which increase the pH.
b. Using the Brønsted–Lowry theory, state whether HCO₃⁻ is acting as an acid or a base.
• Brønsted–Lowry acid = proton donor.
• Brønsted–Lowry base = proton acceptor.
In this equation, HCO₃⁻ accepts a proton (H⁺) from water to form H₂CO₃. Thus, it is acting as a Brønsted–Lowry base.
c. Identify the conjugate base of carbonic acid (H₂CO₃).
H₂CO₃ ⇌ H⁺ + HCO₃⁻
Question 31
Which of the following mixtures is not an acid / conjugate base pair?
A. H₂O / OH⁻.
B. H₂PO₄⁻ / HPO₄²⁻.
C. KH / K.
D. NH₃ / NH₂⁻.
Answer: C. KH / K.
A. Incorrect: H₂O / OH⁻
• H₂O ⇌ H⁺ + OH⁻
• These differ by one proton (H⁺) → they are an acid/conjugate base pair.
B. Incorrect: H₂PO₄⁻ / HPO₄²⁻
• H₂PO₄⁻ ⇌ H⁺ + HPO₄²⁻
• Differ by one proton → also an acid/conjugate base pair.
C. Correct: KH / K
• KH is potassium hydride, which contains the hydride ion (H⁻).
• K is a metal, not a base related to KH by proton gain/loss.
• They do not differ by one proton, so they are not an acid/conjugate base pair.
D. Incorrect: NH₃ / NH₂⁻
• NH₃ ⇌ H⁺ + NH₂⁻
• Differ by one proton → acid/conjugate base pair.
Question 32
Hydrochloric acid is a strong acid, whereas ethanoic acid is a weak acid. What is the difference between a strong acid and a weak acid?
A strong acid completely ionises in water, whereas a weak acid only partially ionises in water.
Example:
HCl → H+ + Cl−(complete)
CH3COOH ⇌ H+ + CH3COO−(partial)
Question 33
Which of the following represents the reaction between zinc powder and a dilute aqueous solution of sulfuric acid?
A. Zn + 2H₂SO₄ → 2ZnS + 2H₂O + 3O₂.
B. 4Zn + H₂SO₄ → 4ZnO + H₂S.
C. Zn + H₂SO₄ → ZnSO₄ + H₂.
D. Zn + H₂SO₄ → ZnH₂ + SO₂ + O₂.
Answer: C. Zn + H₂SO₄ → ZnSO₄ + H₂.
This is a metal + acid → salt + hydrogen reaction. The others show products that are incorrect or chemically impossible for dilute acid (e.g., ZnS, H₂S, SO₂, O₂).
Question 34
Which ions produced by the ionization of phosphoric(V) acid, H₃PO₄, are amphiprotic?
A. HPO₄2⁻ and PO₄3⁻.
B. H₂PO₄⁻ and HPO₄2⁻.
C. HPO₄2⁻ only.
D. H₂PO₄⁻ and PO₄3⁻.
Answer: B. H₂PO₄⁻ and HPO₄2⁻.
An amphiprotic ion can act as both a proton donor and a proton acceptor.
• H₂PO₄⁻ can donate a proton → HPO₄2⁻ or accept a proton → H₃PO₄ → Amphiprotic
• HPO₄2⁻ can donate a proton → PO₄3⁻ or accept a proton → H₂PO₄⁻ → Amphiprotic
Question 35
Which is a 0.001 mol dm⁻3 solution of a weak acid?
| Conductivity | pH | |
| A. | poor | 5 |
| B. | good | 7 |
| C.. | poor | 10 |
| D. | good | 3 |
Answer: A.
Clues about a weak acid (0.001 mol dm⁻3):
• Weak acid → only partially ionizes, so poor conductivity.
• Acidic pH, but not very low — around pH 4–5 for this concentration.
| Option | Conductivity | pH | Explanation |
| A. Correct | Poor | 5 | Fits weak acid — low ionization, slightly acidic |
| B. Incorrect | Good | 7 | Neutral → not acid |
| C. Incorrect | Poor | 10 | Basic → not acid |
| D. Incorrect | Good | 3 | Strong acid (fully ionized) |
Question 36
Which gas in the atmosphere causes the pH of unpolluted rain to be approximately 6?
A. Carbon dioxide.
B. Sulfur dioxide.
C. Oxygen.
D. Nitrogen.
Answer: A. Carbon dioxide.
• Unpolluted rain has a pH of about 5.6–6, slightly acidic. This is due to carbon dioxide (CO₂) dissolving in water to form carbonic acid (H₂CO₃):
CO₂(g) + H₂O(l) ⇌ H₂CO₃(aq) ⇌ H+(aq) + HCO3−(aq)
• Other gases like SO₂ or NO₂ cause acid rain (pH < 5), but these are pollutants, not naturally occurring in unpolluted air.
Question 37
Which row correctly describes 1.0 mol dm⁻3 NaOH (aq)?
| pH | Colour in universal indicator solution | Electrical conductivity | |
| A. | 14 | Purple | Good |
| B. | 10 | Green | Poor |
| C. | 14 | Red | Good |
| D. | 10 | Blue | Poor |
Answer: A.
• NaOH is a strong base, fully ionizes → high [OH⁻].
• pH ≈ 14 (very basic).
• Strong electrolyte → good electrical conductivity.
• Universal indicator in strong base → purple color.
Question 38
a. Define the terms acid and base according to the Brønsted–Lowry theory and state one example of a weak acid and one example of a strong base.
b. Describe two different methods, one chemical and one physical, other than measuring the pH, that could be used to distinguish between ethanoic acid and hydrochloric acid solutions of the same concentration.
c. Black coffee has a pH of 5 and toothpaste has a pH of 8. Identify which is more acidic and deduce how many times the [H⁺] is greater in the more acidic product.
a. Brønsted–Lowry theory:
• An acid is a proton (H⁺) donor.
• A base is a proton (H⁺) acceptor.
Examples:
• Weak acid: Ethanoic acid (CH₃COOH)
• Strong base: Sodium hydroxide (NaOH)
b. To distinguish between ethanoic acid and hydrochloric acid of the same concentration:
• Chemical method: React with magnesium (Mg) and observe the rate of hydrogen gas production.
o HCl (strong acid) reacts faster and more vigorously than CH₃COOH (weak acid).
o Equation:
2HCl + Mg → MgCl₂ + H₂
2CH₃COOH + Mg → (CH₃COO)₂Mg + H₂
• Physical method: Measure electrical conductivity.
o HCl conducts better (fully ionized).
o CH₃COOH conducts poorly (partially ionized).
c.
• Black coffee (pH 5) is more acidic than toothpaste (pH 8).
• The difference in pH = 8 – 5 = 3 units.
• Each pH unit = 10× difference in [H⁺].
103 = 1000
→ [H⁺] in black coffee is 1000 times greater than in toothpaste.
Question 39
Samples of sodium oxide and sulfur trioxide are added to separate beakers of water. Deduce the equation for each reaction and identify each oxide as acidic, basic or neutral.
1. Sodium oxide (Na₂O) with water
Na₂O(s) + H₂O(l) → 2NaOH(aq)
NaOH is a strong base → produces OH⁻ ions. ⇒ Sodium oxide is a basic oxide.
2. Sulfur trioxide (SO₃) with water
SO₃(g) + H₂O(l) → H₂SO₄(aq)
H₂SO₄ is a strong acid → produces H⁺ ions. ⇒ Sulfur trioxide is an acidic oxide.
Question 40
a. The equations of two acid–base reactions are given below.
Reaction A: NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
The reaction mixture in A consists mainly of reactants because the equilibrium lies to the left.
Reaction B: NH₂⁻(aq) + H₂O(l) ⇌ NH₃(aq) + OH⁻(aq)
The reaction mixture in B consists mainly of products because the equilibrium lies to the right.
i. For each of the reactions A and B, deduce whether water is acting as an acid or a base and explain your answer.
ii. In reaction B, identify the stronger base, NH₂⁻ or OH⁻, and explain your answer.
iii. In reactions A and B, identify the stronger acid, NH₄⁺ or NH₃ (underlined), and explain your answer.
b. Describe two different experimental methods to distinguish between aqueous solutions of a strong base and a weak base.
c. Two acidic solutions, X and Y, of equal concentrations have pH values of 2 and 6 respectively.
i. Calculate the hydrogen ion concentrations in the two solutions and identify the stronger acid.
ii. Determine the ratio of the hydrogen ion concentrations in the two solutions X and Y.
a. i.
• Reaction A: NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
Water acts as an acid because it donates a proton (H⁺) to NH₃ to form NH₄⁺.
• Reaction B: NH₂⁻(aq) + H₂O(l) ⇌ NH₃(aq) + OH⁻(aq)
Water acts as an acid because it donates a proton (H⁺) to NH₂⁻ to form NH₃. ii. In reaction B, the stronger base is NH₂⁻.
Reason: The equilibrium lies to the right, showing NH₂⁻ deprotonates water to give NH₃ and OH⁻. → NH₂⁻ is a stronger proton acceptor than OH⁻. (Equivalently, its conjugate acid NH₃ is much weaker than water.)
iii. The stronger acid is NH₄⁺ (vs. NH₃).
Reason: From (ii), NH₂⁻ ≫ NH₃ in basicity, so the conjugate acid of NH₃ (NH₄⁺) is stronger than NH₃ as an acid. (Also, in Reaction A, the products include the stronger acid NH₄⁺, so the equilibrium stays to the left.)
b. Two experimental ways to distinguish solutions of a strong base and a weak base (same concentration):
(Other acceptable: calorimetry of neutralization with a strong acid—strong base gives a larger temperature rise.)
c. Two acids X and Y (same concentration), with pH = 2 and 6
i.
[H⁺]X = 10−2 mol dm−3
[H⁺]Y = 10−6 mol dm−3
→ Stronger acid: X (higher [H⁺], lower pH).
ii. Ratio = `frac{[H^+]_X}{[H^+]_Y}=frac{10^(-2)}{10^(-6)}=frac{10^(-4)}{1}=frac{10000}{1}`
Question 1
What is the order of increasing acidity?
| Acid | pKₐ | Acid | Kₐ |
| HClO | 7.4 | HF | 5.6 × 10⁻⁴ |
| HIO₃ | 0.8 | CH₃CH₂COOH | 1.3 × 10⁻⁵ |
A. HClO < CH₃CH₂COOH < HF < HIO₃
B. HClO < HF < CH₃CH₂COOH < HIO₃
C. HIO₃ < HF < CH₃CH₂COOH < HClO
D. HIO₃ < CH₃CH₂COOH< HF < HClO
Question 2
a. Explain why a 1.0 mol dm⁻3 solution of sodium hydroxide has a pH of 14 whereas 1.0 mol dm⁻3 ammonia solution has a pH of about 12. Use equations in your answer.
b. 20.0 cm3 of a known concentration of sodium hydroxide is titrated with a solution of nitric acid. The graph for this titration is given below.

i. State an equation for the reaction between sodium hydroxide and nitric acid.
ii. Calculate the concentration of the sodium hydroxide solution before the titration.
iii. From the graph determine the volume of nitric acid required to neutralize the sodium hydroxide and calculate the concentration of the nitric acid.
iv. Predict the volume of ethanoic acid of the same concentration as the nitric acid in (b)(iii), required to neutralize 20.0 cm3 of this sodium hydroxide solution.
Question 3
The graph below shows the change in pH when aqueous sodium hydroxide is added to 20 cm³ of aqueous hydrochloric acid.

By reference to the graph:
a. State the [H⁺] before any alkali is added.
b. State by how much the [H⁺] changes after the addition of 20 cm³ of aqueous sodium hydroxide.
c. Determine the volume of the same sodium hydroxide solution needed to neutralize 20 cm³ of aqueous ethanoic acid of the same concentration as the hydrochloric acid.
Question 4
Carbonic acid can be used to treat wasp (an insect) stings.
a. Suggest what this indicates about the nature of wasp stings.
b. Name the type of reaction that occurs.
c. Explain why hydrochloric acid is not used to treat wasp stings.
Question 5
Five unlabelled bottles are known to contain the following 0.10 mol dm⁻³ aqueous solutions:
CH₃COOH, NaCl, NaOH, HCl, NH₃
a. Describe and explain how the pH values of these five solutions could be used to identify them.
b. Experiments were conducted to illustrate some properties of sodium hydrogencarbonate, NaHCO₃.
i. In one experiment some solid NaHCO₃ was added to aqueous NaOH. After stirring the pH decreased to 9. Write a balanced chemical equation for the reaction and explain the decrease in pH.
ii. In another experiment solid NaHCO₃ was added to an aqueous solution of HCl. After stirring the pH increased to 5. Write a balanced equation for the reaction and explain this result.
c. Describe how the two reactions of NaHCO₃ in (b) illustrate the Brønsted–Lowry theory of acids and bases.
Question 6
Which solution is basic at 25 °C?
Kw = 1.0 × 10−14
A. [H+] = 1.0 × 10-3 mol dm-3
B. [OH-] = 1.0 × 10-13 mol dm-3
C. Solution of pH = 4.00
D. [H3O+] = 1.0 × 10-13 mol dm-3
Question 7
The strengths of organic acids can be compared using Ka and pKa values. Which acid is the strongest?
A. Acid A – pKa = 6.6
B. Acid B – pKa = 2.5
C. Acid C – Ka = 1 × 10−5
D. Acid D – Ka = 1 × 10−3
Question 8
What is the pH of 0.001 mol dm-3 NaOH (aq)?
A. 1
B. 3
C. 11
D. 13
Question 9
A 20.00 cm3 solution of the weak monoprotic acid (HA), was titrated against a solution of 0.50 mol dm-3 of sodium hydroxide in which a few drops of indicator had been added. The pH readings were not recorded until 10.00 cm3 of sodium hydroxide had been added.

a. State the volume of sodium hydroxide needed to exactly neutralize the weak acid and hence calculate the amount of sodium hydroxide, in moles, required for neutralization.
b. Write an expression for the dissociation constant, Kₐ, of the weak acid.
c. Calculate a value for the dissociation constant, Kₐ, of the weak acid if the pH of the solution titrated is 2.10.
d. Given the following information about three indicators, state and explain which indicator is the most suitable for determining the end-point of this reaction.
| Indicator | pH range of colour change |
| Methyl red | 4.4–6.2 |
| Cresol red | 7.2–8.8 |
| Alizarin yellow | 10.1–12.0 |
Question 10
When carbon dioxide reacts with water, it forms carbonic acid, H₂CO₃, which ionizes into hydrogencarbonate ions, HCO₃⁻, and hydrogen ions, H⁺, into the sea, decreasing its pH and causing acidification.
A solution of 0.100 mol dm⁻³ H₂CO₃ has a pH of 3.68.
Due to the increasing levels of atmospheric CO₂, the pH of seawater has decreased over 150 years from 8.25 to 8.14. HCO₃⁻ and CO₃²⁻ are the essential components of the carbonate buffer system which regulates the pH of seawater.
CO₂(aq) + CO₃²⁻(aq) + H₂O(l) ⇌ 2HCO₃⁻(aq)
The natural pH of the ocean is determined by the deposition of calcium carbonate in coral reefs against the entry of calcium and carbonate ions into the ocean from weathering of limestone rocks and other minerals on land.
Ca²⁺(aq) + CO₃²⁻(aq) ⇌ CaCO₃(s)
a. Explain, with the aid of appropriate calculations, whether carbonic acid, H₂CO₃, is a strong or weak acid. Assume carbonic acid to be monoprotic in your calculations.
b. Calculate the percentage increase in the concentration of H⁺ ions in the last 150 years.
c. Suggest another environmental problem that can contribute to ocean acidification
Question 11
a. i. Define a Brønsted–Lowry acid.
ii. Deduce the two acids and their conjugate bases in the following reaction:
H₂O (l) + NH₃ (aq) ⇌ OH⁻ (aq) + NH₄⁺ (aq)
iii. Explain why the following reaction can also be described as an acid–base reaction:
F⁻ (g) + BF₃ (g) ⇌ BF₄⁻ (s)
b. Ethanoic acid, CH₃COOH, is a weak acid.
i. Define the term weak acid and state the equation for the reaction of ethanoic acid with water.
ii. Vinegar, which contains ethanoic acid, can be used to clean deposits of calcium carbonate from the elements of electric kettles. State the equation for the reaction of ethanoic acid with calcium carbonate.
Question 12
Which titration curve would occur when a weak acid is added to a strong base?\



Question 13
What is the buffer region in the acid – base titration curves below?

Question 14
Determine the pH of a buffer solution, correct to two decimal places, showing your working, consisting of 10.0 g of CH3COOH and 10.0 g of CH3COONa in 0.250 dm³ of solution. Ka forCH3COOH = 1.8 × 10⁻⁵ at 298 K.
Question 15
Hypochlorous acid, HOCl (aq), is an example of a weak acid.
a. State the expression for the ionic product constant of water, Kw.
b. A household bleach contains sodium hypochlorite, NaOCl (aq), at a concentration of 0.705 mol dm⁻3. The hypochlorite ion, OCl⁻ (aq), is a weak base.
OCl⁻ (aq) + H₂O (l) ⇌ HOCl (aq) + OH⁻ (aq)
i. The pKₐ value of HOCl (aq) is 7.52. Determine the Kb value of OCl⁻ (aq) assuming a temperature of 298 K.
ii. Determine the concentration of OH⁻ (aq), in mol dm⁻3, at equilibrium and state one assumption made in arriving at your answer other than a temperature of 298 K.
iii. Calculate the pH of the bleach.
Question 16
The graph below shows a computer simulation of a titration of 25.0 cm3 of 0.100 mol dm⁻3 hydrochloric acid with 0.100 mol dm⁻3 sodium hydroxide and the pH range of phenol red indicator.

Sketch the graph that would be obtained for the titration of 25.0 cm3 of 0.100 mol dm⁻3 propanoic acid with 0.100 mol dm⁻3 potassium hydroxide using bromophenol blue as an indicator. (The pH range of bromophenol blue can be found in Table 16 of the Data Booklet).

Question 17
The pKa of ethanoic acid is 4.8 at 298 K. Which combination will produce a buffer solution with a pH of 4.8 at 298 K?
A. 20.0 cm3 of 1.0 mol dm⁻3 CH₃COOH and 10.0 cm3 of 1.0 mol dm⁻3 NaOH. B. 20.0cm3 of 1.0 mol dm⁻3 CH₃COOH and 20.0 cm3 of 1.0 mol dm⁻3 NaOH. C. 10.0 cm3 of 1.0 mol dm⁻3 CH₃COOH and 20.0 cm3 of 1.0 mol dm⁻3 NaOH. D. 14.8 cm3 of 1.0 mol dm⁻3 CH₃COOH and 10.0cm3 of 1.0 mol dm⁻3 NaOH.
Question 18
Which indicator is appropriate for the acid–base titration shown below?

A. Thymol blue (pKₐ = 1.5)
B. Methyl orange (pKₐ = 3.7)
C. Bromophenol blue (pKₐ = 4.2)
D. Phenolphthalein (pKₐ = 9.6)
Question 19
What is a possible value of pH at the equivalence point in the titration of a strong acid with a weak base?
A. 11
B. 8
C. 7
D. 5
Question 20
The indicator bromophenol blue, HIn (aq), has a form that is yellow and an In⁻ (aq) form that is blue.
a. Write an equation to show how bromophenol blue acts as an indicator.
b. State and explain the colour of bromophenol blue:
i. On the addition of a strong acid.
ii. At the equivalence point of a titration.
Question 21
Which 1.0 mol dm⁻3 solution has the highest pH?
A. Ammonium chloride.
B. Sulfuric acid.
C. Sodium chloride.
D. Ammonia.
Question 22
10.00 cm3 of 0.01 mol dm-3 nitric acid (HNO₃) is diluted with 90.00 cm³ of water. What is the pH of the resulting solution?
A. 4
B. 3
C. 2
D. 1
Question 23
When the following 1.00 mol dm⁻3 aqueous solutions are listed in increasing order of pH (lowest first), what is the correct order?
A. HNO₃ < HCOOH < NH₃ < Ba(OH)₂.
B. NH₃ < Ba(OH)₂ < HCOOH < HNO₃.
C. Ba(OH)₂ < HCOOH < NH₃ < HNO₃.
D. HNO₃ < HCOOH < Ba(OH)₂ < NH₃.
Question 24
The amino acid alanine has the molecular structure NH₂CH(CH₃)COOH. Which of the following species represents its conjugate acid?
A. ⁺NH₃CH(CH₃)COOH
B. ⁺NH₃CH(CH₃)COOH₂⁺
C. ⁺NH₃CH(CH₃)COO⁻
D. NH₂CH(CH₃)COO⁻
Question 25
Which is a conjugate acid–base pair according to the Brønsted–Lowry theory?
CH₂ClCOOH (aq) + H₂O (l) ⇌ CH₂ClCOO⁻ (aq) + H₃O⁺ (aq)
A. H₂O / H₃O⁺
B. H₂O / CH₂ClCOO⁻
C. CH₂ClCOO⁻ / H₃O⁺
D. CH₂ClCOOH / H₂O
Question 26
A sample of benzenecarboxylic acid solution, C₆H₅COOH (aq), is diluted at constant temperature. Which diagram shows how the pH of the acid changes as it is diluted? [V is the volume of water added.]


Question 27
Under suitable conditions, NH₄I and NaNH₂ react as follows:
NH₄I + KNH₂ → KI + 2NH₃
Which term best describes the above reaction?
A. Reduction–oxidation reaction.
B. Displacement reaction.
C. Brønsted–lowry acid–base reaction.
D. Substitution reaction.
Question 28
a. Outline the Brønsted–Lowry theory of acids and bases.
b. Write a chemical equation, including state symbols, to show why rain water is slightly acidic.
c. The carbonate ion is a conjugate base of the hydrogencarbonate ion, HCO₃⁻. Define the term conjugate base.
d. Using appropriate chemical equations, show that the hydrogencarbonate ion is amphiprotic and can act as a proton donor and a proton acceptor.
Question 29
The equation for the reaction that occurs when ammonia gas dissolves in water is shown below:
NH₃(g) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
a. State how the equation indicates that ammonia is a base.
b. State how the equation indicates that ammonia is a weak base.
c. Identify which pH value is approximately correct for ammonia solution.
pH: 1 3 7 11 13
Question 30
Sodium hydrogencarbonate dissolves in water forming an alkaline solution according to the following ionic equilibrium:
HCO₃⁻(aq) + H₂O(l) ⇌ H₂CO₃(aq) + OH⁻(aq)
a. Why is the solution alkaline?
b. Using the Brønsted–Lowry theory, state, with a brief explanation, whether the HCO₃⁻ ion is behaving as an acid or as a base.
c. Identify the conjugate base of carbonic acid, H₂CO₃.
Question 31
Which of the following mixtures is not an acid / conjugate base pair?
A. H₂O / OH⁻.
B. H₂PO₄⁻ / HPO₄²⁻.
C. KH / K.
D. NH₃ / NH₂⁻.
Question 32
Hydrochloric acid is a strong acid, whereas ethanoic acid is a weak acid. What is the difference between a strong acid and a weak acid?
Question 33
Which of the following represents the reaction between zinc powder and a dilute aqueous solution of sulfuric acid?
A. Zn + 2H₂SO₄ → 2ZnS + 2H₂O + 3O₂.
B. 4Zn + H₂SO₄ → 4ZnO + H₂S.
C. Zn + H₂SO₄ → ZnSO₄ + H₂.
D. Zn + H₂SO₄ → ZnH₂ + SO₂ + O₂.
Question 34
Which ions produced by the ionization of phosphoric(V) acid, H₃PO₄, are amphiprotic?
A. HPO₄2⁻ and PO₄3⁻.
B. H₂PO₄⁻ and HPO₄2⁻.
C. HPO₄2⁻ only.
D. H₂PO₄⁻ and PO₄3⁻.
Question 35
Which is a 0.001 mol dm⁻3 solution of a weak acid?
| Conductivity | pH | |
| A. | poor | 5 |
| B. | good | 7 |
| C.. | poor | 10 |
| D. | good | 3 |
Question 36
Which gas in the atmosphere causes the pH of unpolluted rain to be approximately 6?
A. Carbon dioxide.
B. Sulfur dioxide.
C. Oxygen.
D. Nitrogen.
Question 37
Which row correctly describes 1.0 mol dm⁻3 NaOH (aq)?
| pH | Colour in universal indicator solution | Electrical conductivity | |
| A. | 14 | Purple | Good |
| B. | 10 | Green | Poor |
| C. | 14 | Red | Good |
| D. | 10 | Blue | Poor |
Question 38
a. Define the terms acid and base according to the Brønsted–Lowry theory and state one example of a weak acid and one example of a strong base.
b. Describe two different methods, one chemical and one physical, other than measuring the pH, that could be used to distinguish between ethanoic acid and hydrochloric acid solutions of the same concentration.
c. Black coffee has a pH of 5 and toothpaste has a pH of 8. Identify which is more acidic and deduce how many times the [H⁺] is greater in the more acidic product.
Question 39
Samples of sodium oxide and sulfur trioxide are added to separate beakers of water. Deduce the equation for each reaction and identify each oxide as acidic, basic or neutral.
Question 40
a. The equations of two acid–base reactions are given below.
Reaction A: NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
The reaction mixture in A consists mainly of reactants because the equilibrium lies to the left.
Reaction B: NH₂⁻(aq) + H₂O(l) ⇌ NH₃(aq) + OH⁻(aq)
The reaction mixture in B consists mainly of products because the equilibrium lies to the right.
i. For each of the reactions A and B, deduce whether water is acting as an acid or a base and explain your answer.
ii. In reaction B, identify the stronger base, NH₂⁻ or OH⁻, and explain your answer.
iii. In reactions A and B, identify the stronger acid, NH₄⁺ or NH₃ (underlined), and explain your answer.
b. Describe two different experimental methods to distinguish between aqueous solutions of a strong base and a weak base.
c. Two acidic solutions, X and Y, of equal concentrations have pH values of 2 and 6 respectively.
i. Calculate the hydrogen ion concentrations in the two solutions and identify the stronger acid.
ii. Determine the ratio of the hydrogen ion concentrations in the two solutions X and Y.